DIAGNOSIS AND MANAGEMENT OF PROSTATITIS
ANTHONY J. SCHAEFFER
Department of Urology, Northwestern University Medical School, Chicago, Illinois, USA
ABSTRACT
Prostatitis is a common syndrome characterized by infection, pain and/or inflammation of the prostate or surrounding tissues. The diagnosis requires a careful history, physical exam, and localization of inflammation and/or infection to the prostate. Bacterial prostatitis is uncommon and characterized by acute or recurrent infection of the bladder, which usually responds to antimicrobial therapy. Chronic pelvic pain syndrome is common and characterized by pelvic pain, no infection and varying degrees of prostate inflammation. Because the etiology is unknown, empiric therapy is utilized and only moderately successful. Asymptomatic prostatitis is detected in the course of evaluation for BPH, prostate cancer or infertility and should only be treated if the underlying condition warrants therapy.
Key words:
prostate, prostatitis, inflammation, infection, prostatodynia
Braz J Urol, 26: 122-131, 2000
INTRODUCTION
Prostatitis syndrome is one of the most common entities encountered in clinical practice. It is estimated that up to 50% of adult men experience complaints of symptoms of prostatitis at some time in their lives. The symptoms of prostatitis may mimic the symptoms of bladder outlet obstruction from prostatic hyperplasia, which may further confuse the clinician. The differentiation of infectious from non-infectious prostatitis is essential and requires careful attention to details of specimen collection. New drugs have improved therapy and hold promise for better results in the future.
EPIDEMIOLOGY/CLASSIFICATION
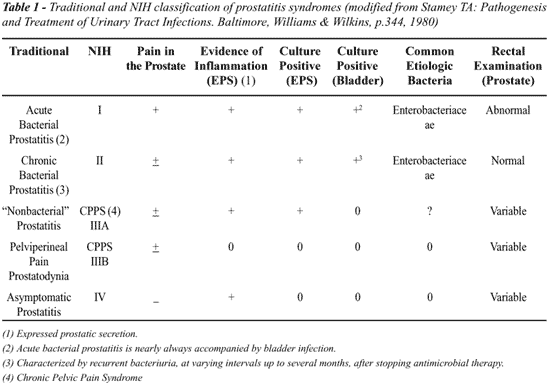
The diagnosis of prostatitis is made at an estimated two million outpatient visits each year in the United States. Prostatitis is the most common urologic diagnosis for men under the age of fifty, and the third most common urologic diagnosis for those over fifty (1). The epidemiological literature quotes anywhere from 9-50% of all men will be diagnosed with prostatitis at some time in their life (2,3). Traditionally, prostatitis has been classified as acute or chronic bacterial, chronic nonbacterial, or prostadynia with the latter two categories comprising roughly 90% of all prostatitis cases (Table-1). Recently, the National Institutes of Health Consensus Conference on Prostatitis developed a more specific classification scheme for prostatitis. Categories I and II (i.e. acute or chronic bacterial prostatitis) are based on symptoms and identification of bacteria in the urine or expressed prostate secretions (EPS) respectively. Chronic Pelvic Pain Syndrome (CPPS), Category III, is based on symptoms of chronic pelvic pain and Category IV refers to asymptomatic patients with coincidental finding of prostate inflammation in patients undergoing evaluation for benign prostatic hyperplasia, prostate cancer, or infertility.
DIAGNOSIS
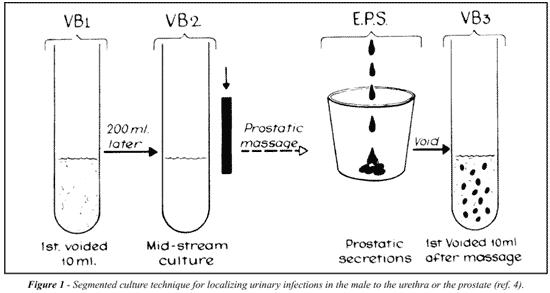
The cornerstone of diagnosis and treatment for prostatitis remains appropriate localization of inflammation and infection to the prostate as described by Meares et al. (4). Figure-1 describes the four-glass test of sequential voided urine collected both before and after prostatic massage. The urine and expressed prostatic secretion (EPS) are examined microscopically to identify inflammation and are cultured to determine the type, number and antimicrobial sensitivity of the pathogens.
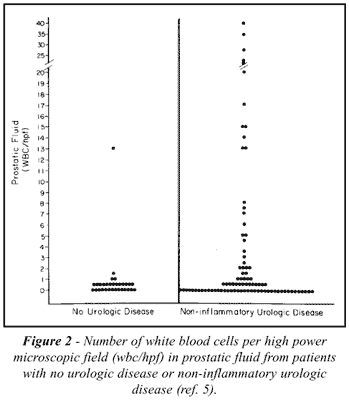
To define prostatitis, the degree of inflammatory
changes that can be found in normal prostatic fluid must first be determined.
Available data suggest that white blood cells are rarely present in normal
prostatic fluid. Schaeffer et al. (5) studied 119 consecutive patients
with no history, symptoms, or physical findings (excluding prostatic fluid
evaluation) of urinary tract inflammation, normal prostate gland by digital
examination, and fewer than two white blood cells per high-power field
in the first 10 ml of voided urine and no, or insignificant, growth on
urine culture (Figure-2). Of these patients, 31 were judged to have no
urologic disease and they had prostatic fluid containing 0.7 ±
0.41 white blood cells per high-power field (mean ± standard error
of mean), and 88, with a variety of noninflammatory urologic diseases,
had 3.8 ± 0.83 white blood cells per high-power field in the prostatic
fluid. There were two white blood cells or less per high-power field observed
in 97 percent of the patients with no urologic disease and in 75 percent
with noninflammatory urologic disease and normal prostate glands by digital
examination. Only 13 of the 119 patients in these two groups had ten white
blood cells or more per high-power field. Blacklock (6) and Anderson &
Weller (7) have reported similar results. It appears therefore that clinically
significant inflammation is present when prostatic fluid contains ten
or more white blood cells per high-power field. The white blood cell count
in prostatic secretions also rises significantly in healthy men for several
hours after sexual intercourse and ejaculation.
Acute bacterial prostatitis is diagnosed
by identification of bacteria in the urine of a patient with an acute,
septic urinary tract infection. Chronic bacterial prostatitis is diagnosed
by culture of any uropathogen in the EPS in the absence of urethral pathogens
(VB1 urine culture), or a cultured EPS or VB3 bacterial count at least
ten times greater than cultured VB1 or VB2 urine.
Chronic pelvic pain syndrome is diagnosed
by the complaint of pelvic pain for a total of 3 months within the last
6 months. Patients are excluded if they have any of the conditions listed
on Table-2.

Patients with asymptomatic prostatitis are
identified by elevated levels of white blood cells in the EPS (for example
to determine if prostatic inflammation is associated with an elevated
prostate specific antigen level) or ejaculate (in a patient being evaluated
for infertility).
PATHOGENESIS
The
pathogenesis of bacterial prostatitis is unknown. Presumably ascending
urethral infection after a vaginal or rectal inoculation of the urinary
meatus during sexual intercourse plays an important role. As such, the
prostatitis may represent an extension or a complication of urethritis.
Evidence of reflux of urine into the prostatic ducts has come from crystallographic
analysis of prostatic calculi where constituents found in urine, but foreign
to prostatic secretions, were discovered. Direct bacterial inoculation
from the rectum or hematogenous or lymphatic spread to the prostate may
also occur.
Bacterial prostatitis is caused by the usual
uropathogens. Escherichia coli and other members of the Enterobacteriaceae
family, such as Klebsiella and Proteus species, predominate. Pseudomonas
and Enterococcus faecalis are less common. Mixed infections involving
two or more strains or classes of microorganisms are not uncommon (8).
The role of gram-positive bacteria, other
than enterococci, as pathogens in prostatitis is controversial. Although
uncommon, chronic bacterial prostatitis caused by Staphylococcus aureus
has been documented, usually as a consequence of a hospital- acquired
catheter-associated infection. The etiologic role of other gram-positive
bacteria (e.g. Staphylococcus epidermidis and saprophyticus, micrococci,
non-group D streptococci, and diphtheroids) is doubtful. These organisms
are considered skin inhabitants, existing as urethral commensals rather
than true pathogens. Furthermore, they do not cause relapsing recurrent
urinary tract infections in untreated patients.
Transrectal ultrasonography detects prostatic
calculi in 75 percent of middle-aged men and nearly l00 percent of elderly
men (8). Moreover, transrectal ultrasonography is capable of demonstrating
stones in as many as 70 percent of men who have no other radiologic signs
of prostatic stones (9). Prostatic stones usually are tiny and occur in
small clusters; at times; however, the stones may be large and extensively
involve the prostate. Bacteria do not usually colonize these calculi and
cause no harm provided they remain confined to the prostate. In certain
men with prostate calculi and recurrent urinary tract infections, however,
the stones have been shown to become colonized and to be the source of
bacterial persistence (10,11). Similar to colonized renal calculi, prostatic
stones can be permeated with bacteria that are protected from the action
of antimicrobial agents. Because prostatic calculi are exceedingly common
in adult men, and because these stones can become colonized during or
after formation, it is plausible that unrecognized colonized stones are
often responsible for the failure of antimicrobial therapy to cure chronic
bacterial prostatitis.
The pathophysiology of inflammatory CPPS
may involve the intraprostatic reflux of urine inciting a chemical inflammatory
response (12); however, other factors such as an autoimmune mediated process
may also be responsible (13). Past studies implicated Ureaplasma, Mycobacterium,
or, most commonly, Chlamydia as pathogens responsible for the development
of inflammatory CPPS, yet recent well designed studies have found no evidence
that these pathogens are involved in the etiology of inflammatory CPPS
(14,15). The common urodynamic findings in patients with inflammatory
CPPS include synergistic voiding (normal relaxation of the pelvic floor
musculature during the act of voiding) with incomplete relaxation of the
bladder neck and prostatic urethra. The urinary peak flow is usually decreased
with the urethral pressure profile demonstrating an increased maximal
urethral closing pressure at rest (16). It is felt that the high pressure
generated from smooth muscle spasm of the bladder neck and prostatic urethra
during the act of voiding leads to intraprostatic reflux of urine.
The pathophysiology of non-inflammatory
CPPS involves a poorly understood chronic myofascial pain complex that
may be related to a tension myalgia of the pelvic floor musculature. Pathologically
there are no specific findings and no evidence of inflammation in the
prostatic gland. Accordingly, urodynamic findings are nondistinguishable
from those of patients with inflammatory CPPS.
The pathophysiology of asymptomatic prostatitis
is unknown and probably multifactorial. Factors that may cause symptomatic
prostatitis (IIIA), e.g. infectious agents that are difficult to culture
or an autoimmune response, may play a role in the pathogenesis of asymptomatic
prostatitis.
MANAGEMENT
I - Acute
bacterial prostatitis
Acute bacterial prostatitis usually presents
in a relatively young man with dramatic onset of fever, malaise, low back
or perineal pain, and myalgia for several days prior to onset of symptoms
of urinary frequency, dysuria, urgency, and varying degrees of bladder
outlet obstruction. Palpation usually but not always reveals a tender,
hard, irregular prostate that is warm to touch. Prostatic massage should
be avoided because of the risk of bacteremia, but gentle pressure on the
prostate may induce copious amounts of purulent prostatic secretions.
Since cystitis usually accompanies acute bacterial prostatitis, the responsible
bacterial pathogen can be isolated from bladder urine. Escherichia coli
and other members of the Enterobacteriaceae family account for 95% of
cases; Pseudomonas and enterococcus (Streptococcus faecalis) microorganisms
are less common. Serum chemistry reveals an elevated white blood cell
count.
General
Therapy
Most patients are quite toxic and may require
admission to the hospital. Supportive measures such as analgesics, antipyretics,
hydration, bed rest, and stool softeners should be instituted. If the
patient cannot urinate, urethral catheterization may further exacerbate
the prostatic inflammation and lead to complications such as acute epididymitis.
Therefore use of a small urethral catheter or a suprapubic tube are advised.
Antimicrobial
Therapy
Patients with acute bacterial prostatitis
usually respond dramatically to antimicrobial drugs that normally do not
achieve therapeutic levels in prostatic fluid. This is because the inflamed
prostate permits diffusion of drugs from the bloodstream to the prostate.
If the patient is septic, we obtain blood cultures and administer gentamicin
sulfate or tobramycin sulfate, 3 to 5 mg per kg of body weight per day,
divided into three intramuscular or intravenous doses, plus ampicillin,
1 gram administered intravenously every 6 hours or an intravenous fluoroquinolone
such as ciprofloxacin (Cipro) 200-400 mg every 12 hours or ofloxacin (Floxin)
200-400 mg every 12 hours. If the patient can take oral antimicrobial
agents, a fluoroquinolone such as ciprofloxacin (Cipro) 500 mg every 12
hours, ofloxacin (Floxin) 300 mg every 12 hours, norfloxacin (Noroxin)
400 mg every 12 hours, lomefloxacin (Maxaquin) 400 mg every day or enoxacin
(Penetrex) 400 mg every 12 hours can be utilized. Although two weeks of
therapy is probably adequate, four weeks is preferred to ensure that all
bacteria are eliminated from the prostate.
Operative
Therapy
Surgical intervention is generally not indicated
for acute bacterial prostatitis. Prostatic abscess is a rare complication
that should be suspected in patients whose symptoms and clinical courses
do not respond to appropriate antimicrobial therapy. If a large, localized,
tender, fluctuant mass is palpated within the prostate, ultrasound or
computed tomography and perineal or transurethral drainage should be performed.
If the prostate gland is hard, several months may be required before it
returns to normal consistency. Granulomatous prostatitis in the absence
of tuberculosis and rare mycotic infection of the prostate is a histologic
stage of resolving acute bacterial prostatitis, which is usually detected
as a local area of prostatic induration suspicious of carcinoma. Except
for exclusion of carcinoma, no special therapy is warranted.
II - Chronic
Bacterial Prostatitis
Chronic bacterial prostatitis is characterized
by relatively asymptomatic periods in between episodes of recurrent bacteriuria.
The infection is caused by small numbers of bacteria in the prostatic
fluid and is very difficult to eradicate with most antimicrobial therapy.
The common pathogens are the Enterobacteriaceae and species of Pseudomonas.
Enterococcus (Streptococcus faecalis) is also a definite cause of chronic
bacterial prostatitis. Other gram-positive organisms have been implicated
much less frequently and rarely cause recurrent bacteriuria. Mixed infections
involving two or more strains or classes of microorganisms are uncommon.
Hematospermia and painful ejaculation occur
infrequently. Prostate examination is nondiagnostic.
General
Therapy
Appropriate oral antimicrobial therapy usually
controls the acute episode of cystitis. Hot sitz baths and antipyretics
are also helpful. Septic episodes requiring hospitalization and parenteral
therapy occur rarely.
Antibacterial
Therapy
Cure of bacterial prostatitis appears to
correlate best with the level of antimicrobial drug in the prostatic fluid
rather than its level in serum or prostatic tissue. Trimethoprim and the
fluoroquinolones do diffuse into prostatic fluid and have the best documented
success in curing chronic bacterial prostatitis due to susceptible pathogens.
Long-term therapy (8-12 weeks) appears to be more effective than short-term
therapy (2 weeks) in achieving bacteriologic cures. The following recommendations
are made for treatment of nonazotemic men with documented culture susceptible
pathogens infecting the prostate:
1)- TMP-SMX (Septra or Bactrim), one double-strength tablet (160 mg of
TMP and 800 mg of SMX) orally twice daily for 12 weeks; or
2)- Trimethoprim (Proloprim or Trimpex), two tablets (100 mg each) orally
twice daily for 12 weeks; or
3)- Ciprofloxacin (Cipro), 250 mg every 12 hours for 4 weeks; or
4)- Enoxacin (Penetrex), 400 mg every 12 hours for 4 weeks; or
5)- Lomefloxacin (Maxaquin), 400 mg every day for 4 weeks; or
6)- Norfloxacin (Noroxin), 400 mg every 12 hours for 4 weeks; or
7)- Ofloxacin (Floxin), 200 mg every 12 hours for 4 weeks.
Specific therapy must always be tailored
to meet the individual patient’s needs and drug tolerance (see also
the manufacturer’s official directive in the use of these agents.)
Patients not cured by antimicrobial therapy can be kept comfortable and
abacteriuric by use of continuous low-dose suppressive daily therapy with
an appropriate oral antimicrobial agent such as nitrofurantoin (50 mg
capsule) or trimethoprim-sulfamethoxazole (TMP-SMX; a single, regular
strength tablet each day). Bacteriuria will usually recur following cessation
of therapy.
Operative
Therapy
Transurethral resection of the prostate
is the only alternative, short of radical prostatectomy, for surgical
management of bacterial prostatitis. However, transurethral prostatectomy
can be curative only if all foci of infected tissue and calculi are removed.
Since most inflammation in chronic prostatitis occurs in the periphery
of the gland and all the ducts from the peripheral zone empty into the
urethra distal to the verumontanum, radical transurethral resection with
removal of all foci of infected stones and tissues is difficult to achieve
and carries a high risk of urinary incontinence. Approximately one-third
of patients with well-documented bacterial prostatitis have been cured
by this technique.
III -
Chronic Pelvic Pain Syndrome (CPPS)
Diagnosis and treatment of this syndrome
is controversial. CPPS (in the clinical setting, “prostatitis”)
is most frequently synonymous with pain in the pelvic or perineum frequently
associated with urinary urgency frequency dysuria or poor urine flow.
The syndrome has been called prostatodynia because the symptoms have been
judged to be of prostatic origin. However, many patients are probably
unable to differentiate prostatic pain from pelvic or perineal symptoms.
CPPS therefore is a more appropriate term to describe this condition.
Evidence of inflammation in the EPS is variable suggesting that different
factors (e.g. infections, inflammation, musculo-skeletal) may contribute
to the etiology and pathogenesis of the syndrome. It is not surprising
therefore that a wide variety of agents have been used to treat patients
with CPPS and that the outcomes are so varied.
III.A
- Inflammatory CPPS (Nonbacterial Prostatitis)
Nonbacterial prostatitis is about eight
times more common than bacterial prostatitis. The clinical significance
of evidence for prostate inflammation, particularly in asymptomatic patients,
has been questioned. However, recognition that identifiable groups of
patients (such as those with infertility, i.e., category IV) have significant
increased leukocyte counts indicates that nonbacterial prostatitis may
be indicative of an underlying disease.
General
Therapy
Since the etiology is unknown, treatment
is empiric and often unrewarding. Two important conditions should be considered
in a differential diagnosis, interstitial cystitis and carcinoma in-situ
of the bladder. Thus for selected patients it may be reasonable to obtain
urine specimens for cytology and perform endoscopic evaluation under anesthesia
(17). Some experts advocate that an initial trial of antimicrobials is
warranted for inflammatory CPPS. The antimicrobial regimen consists of
an initial 28-day course of a fluoroquinolone or a six-week trial of TMP/SMX
as upwards of 40% of these patients will demonstrate clinical improvement
(18). This initial trial of antimicrobials is supported by the fact that
men with inflammatory CPPS demonstrate a higher incidence of significant
bacterial infection on prostate biopsy culture than noninflammatory CPPS
patients (19). If Chlamydia or Ureaplasma species are likely causes of
urethritis associated with prostatitis, we recommend a clinical trial
with minocycline (Minocin), 100 mg orally twice daily for 10 days. Unless
the response is favorable, further treatment is probably not indicated.
Continued empiric administration of other antimicrobial drugs is almost
invariably ineffective and engenders considerable expense, anxiety, and
dissatisfaction. Instead, efforts should be made to educate the patient
with a frank discussion about the unknown and probably noninfectious etiology
of the condition and efforts to relieve symptoms. Videourodynamics may
be obtained in order to exclude other diagnoses such as primary bladder
neck obstruction and pseudodyssynergia of the external urethral sphincter,
both of which are common voiding dysfunction conditions in men less than
fifty years of age misdiagnosed with CPPS (20). Several treatment options
may be employed for inflammatory CPPS including pharmacological alpha
blockade of the bladder neck, nonsteroidal anti-inflammatory agents, warm
sitz baths, prostatic massage, biofeedback and transurethral microwave
thermotherapy of the prostate. We generally recommend hot sitz baths for
symptomatic flare-ups. Many patients obtain symptomatic relief after short
courses of anti-inflammatory agents such as ibuprofen (Motrin), 400 to
600 mg orally three or four times daily. Patients with obstructive voiding
symptoms may benefit from therapy with an alpha-blocker such as terazosin
(Hytrin) 5-mg orally once daily, doxazosin (Cardura) 4-mg orally once
daily, or tamsulosin hydrochloride (Flomax) 0.4 mg once daily. This treatment
has been reported to achieve a clinical success rate of approximately
58% (21), yet better prospective data and the use of standardized diagnostic
criteria need to be employed in future studies. All patients should undergo
urodynamics prior to the initiation of treatment in order to confirm the
diagnosis of CPPS. Concomitant use with beta-blockers or verapamil will
increase the sensitivity of alpha 1 induced postural hypotension. The
antihypertensive effect of clonidine is decreased with the use of an alpha
1 blocker. The cardiovascular adverse effects of hypotension and palpitations
occur in approximately 4% of all patients. Headache and dizziness may
occur in 5-9% of patients on long term therapy. Irritative voiding symptoms
may respond to therapy with anticholinergics, such as propantheline (Pro-Banthine)
15 mg orally, four times daily or oxybutynin chloride (Ditropan), 5 mg
orally, two or three times daily. Oxybutynin chloride (Ditropan) is an
anticholinergic agent that inhibits the muscarinic action of acetylcholine
on smooth muscle. Clinically, oxybutynin exhibits an antispasmodic effect
on detrusor smooth muscle thus diminishing involuntary bladder contractions
associated with the CPPS in some patients. Normal starting dosage is 5
mg every six hours for symptomatic relief with titration to a dose of
10 mg. Contraindications include hypersensitivity to the drug, acute closure
glaucoma, urinary retention, and intestinal atony. The most common side
effects are dry mouth, flushing, and headache.
Tolterodine tartrate (Detrol) is a novel
muscarinic receptor antagonist that inhibits detrusor smooth muscle in
similar fashion as oxybutynin. The apparent advantage of this medication
over oxybutynin is twofold: 1) early studies reveal it is as efficacious
as oxybutynin, yet better tolerated with less side effects because of
its higher selectivity for bladder detrusor smooth muscle (22), and 2)
it is dosed just two times a day as compared to oxybutynin which is dosed
four times a day. Recommended starting dose is 1 mg with titration to
2 mg as needed. Contraindications to the drug are the same as those for
oxybutynin. The cost of tolterodine may be considered a disadvantage,
as it is not generic with retail cost approaching $1 per tablet.
Occasionally, therapeutic prostatic massage
and dietary restrictions regarding the use of alcoholic beverages, coffee,
and spicy foods are beneficial.
Transurethral microwave thermotherapy is
reserved for treatment of refractory inflammatory CPPS patients. Limited
success in symptom relief has been reported with this modality. Standard
Procedure: transurethral placement of a microwave applicator into the
prostatic urethra is performed under direct vision, and the periurethral
prostatic tissue is heated to a temperature of 45-60°C for approximately
one hour. The tissue hyperthermia induced by the microwaves (frequency
of 915-2450 MHz) theoretically ablates sensory neural components of the
prostate thus explaining symptom relief in some patients (23). Further
work is needed to elucidate the effects of thermotherapy on prostatic
tissue.
III.B
- Noninflammatory CPPS (Prostatodynia)
The term prostatodynia has been suggested
for men with symptoms that mimic prostatitis, especially “prostatic
pain,” but who have negative cultures and no evidence of inflammation
in the expressed prostatic secretions. Although some of these symptoms
may be of prostatic origin, the term is misleading if the patient’s
assessment of the etiology of his discomfort is inaccurate. Musculoskeletal
abnormalities are probably responsible for much of the symptomatology.
Some patients with this syndrome have apparent functional obstruction
in the bladder neck and prostatic urethra. These patients may respond
favorably to therapy with an alpha-blocking agent such as those listed
above, once daily at bedtime. Other patients with apparent tension myalgia
of the pelvic floor respond best to treatment with warm sitz baths diathermy,
muscle relaxants, and physiotherapy, with or without the use of diazepam
(Valium), 5 mg orally three times daily. Some patients have emotional
disturbances that benefit from psychiatric consultation.
There is no evidence to support alcohol
intake, caffeine, or tobacco use as risk factors for prostatitis. However,
animal studies suggest that a daily intake of dietary soy protein may
play a protective role against the development of prostatitis (24).
Prostatic massage may be utilized for the
CPPS patient with a congested prostate from sexual inactivity. Firm digital
pressure applied to the prostate two to three times each week has been
anecdotally proven to relieve symptoms in some patients.
Biofeedback has been used for various dysfunctional
voiding disorders with success rates reported as high as 70% (25). Biofeedback
involves training the patient to selectively contract and relax the muscles
of the pelvic floor on a voluntary basis in order to eventually use this
technique for the interruption of pelvic myofascial pain attacks associated
with CPPS. In addition, the patient undergoes a bladder-training program
that involves a progressive increase in the interval between voids to
no less than four hours. Biofeedback involves patient recognition and
eventual correction of the symptoms associated with CPPS. This appears
to be a promising treatment protocol for patients with CPPS, yet prospective
studies involving pre- and post treatment urodynamics, post void residuals,
and voiding diaries are needed.
The sequelae of CPPS include the potential
for male factor infertility, and an association with psychiatric illness
that should not be overlooked by the physician. Treatment refractory CPPS
has a proven sickness impact on quality of life similar to that of patients
with a history of myocardial infarction, angina, and Crohn’s disease
(26). Berghuis et al. (27) suggest that as many as 43% of CPPS patients
suffer from some form of significant psychological distress from their
condition.
IV - Asymptomatic
Prostatitis
Therapy for asymptomatic prostatitis is
only recommended if the cofactor e.g. infertility, elevated prostate specific
antigen is judged to be potentially caused by inflammation. Empiric antimicrobial
therapy or anti-inflammatory drugs listed above may reduce inflammation
and resolve the clinical problem.
CONCLUSION
Prostatitis is a common problem with multiple etiologies. Less than 10% of men have bacterial prostatitis. Acute bacterial prostatitis presents as an acute, serious event and usually responds to prompt antimicrobial therapy. Chronic bacterial prostatitis is always associated with recurrent urinary tract infections. Bacterial localization studies and long term antimicrobial therapy can cure two-thirds of these patients. Chronic Pelvic Pain Syndrome is the most common type of prostatitis and most likely is caused by a variety of autoimmune, inflammatory and muscular disorders of the prostate and other pelvic organs. Most current management is empiric and of limited benefit but research suggests that exciting progress and therapies are forthcoming.
REFERENCES
- 1. Collins MN, Stafford RS, O’Leary MP, Barry MJ: How common is prostatitis? A national survey of physician visits. J Urol, 159: 1224-1228, 1998.
- Stamey TA: Pathogenesis and Treatment of Urinary Tract Infections. Baltimore, Williams & Wilkins, 1980.
- Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ: Prevalence of a physician – assigned diagnosis of prostatitis: The Olmsted county study of urinary symptoms and health status among men. Urology, 51: 578-584, 1998.
- Meares EM, Stamey TA: Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest. Urol., 5: 492, 1968.
- Schaeffer AJ, Wendel EF, Dunn JK, Grayhack JT: Prevalence and significance of prostatic inflammation. J Urol, 125: 215-219, 1981.
- Blacklock NJ: Some Observations on Prostatitis.In: Williams DC, Briggs MH, Stanford M (eds), Advances in the Study of the Prostate. London, Williams Heinemann Medical Books Ltd., pp. 37-55, 1969.
- Anderson RU, Weller C: Prostatic secretion leukocyte studies in non-bacterial prostatitis (prostatosis). J Urol, 121: 292-294, 1979.
- Lim DJ, Schaeffer AJ: Prostatitis Syndromes. AUA Update Series, Volume XII: Lesson 1, 1993.
- Peeling WB, Griffiths GJ: Imaging of the prostate by ultrasound. J Urol, 132: 217-224, 1984.
- Meares EM Jr: Infection stones of the prostate gland, laboratory diagnosis and clinical management. Urology, 4: 560-566, 1974.
- Eykyn S, Bultitude MI, Mayo ME: Prostatic calculi as a source of recurrent bacteriuria in the male. Br J Urol, 46: 527-532, 1974.
- Kirby RS, Lowe D, Bultitude MI, Shuttleworth KE: Intra-prostatic urinary reflux: An etiological factor in abacterial prostatitis. Br J Urol, 54: 729, 1982.
- Alexander RB, Brady F, Ponniah S: Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology, 50: 893-899, 1997.
- Doble A, Thomas BJ, Walker MM, Harris JR, Witherow RO, Taylor-Robinson D: The role of chlamydia trachomatis in chronic abacterial prostatitis: a study using ultrasound guided biopsy. J Urol, 141: 332-333, 1989.
- Shortliffe LM, Sellers RG, Schachter J: The characterization of nonbacterial prostatitis: search for an etiology. J Urol, 148: 1461-1466, 1992.
- Meares EM: Prostatitis and Related Disorders. Campbell’s Urology, ed 7. Philadelphia, WB Saunders, pp. 615-630, 1997.
- Miller JL, Rothman I, Bavendam TG, Berger RE: Prostatodynia and interstitial cystitis: one and the same? Urology, 45: 587-590, 1995.
- Nickel JC: Prostatitis: Myths and realities. Urology, 51: 362-366, 1998.
- Berger RE, Krieger JN, Rothman I, Muller CH, Hillier SL: Bacteria in the prostate tissue of men with idiopathic prostatic inflammation. J Urol, 157: 863-865, 1997.
- Kaplan SA, Santarosa RP, D’Alisera PM, Fay BJ, Ikeguchi EF, Hendricks J, Klein L, Te AE: Pseudodyssynergia (Contraction of the external sphincter during voiding) misdiagnosed as chronic nonbacterial prostatitis and the role of biofeedback as a therapeutic option. J Urol, 157: 2234-2237, 1997.
- Neal DE, Moon TD: Use of terazosin in prostadynia and validation of a symptom score questionnaire. Urology, 43: 460-465, 1994.
- Abrams P, Freeman R, Anderstrom C, Mattiasson A: Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. Br J Urol, 81: 801-10, 1998.
- Nickel JC, Sorensen R: Transurethral microwave thermotherapy for nonbacterial prostatitis: a randomized double-blind sham controlled study using new prostatitis specific assessment questionnaires. J Urol, 155: 1950-1955, 1996.
- Sharma OP, Adlercreutz H, Strandberg JD: Soy of dietary source plays a preventative role against the pathogenesis of prostatitis in rats. J Ster Biochem & Molec Bio, 43: 557-564, 1992.
- Hellstrom AL, Hjalmos K, Jodal U: Rehabilitation of the dysfunctional bladder in children: method and 3 year follow-up. J Urol, 138: 847, 1987.
- Wenninger K, Heiman JR, Rothman I, Berghuis JP, Berger RE: Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol, 155: 965-968, 1996.
- Berghuis JP, Heiman JR, Rothman I, Berger RE: Psychological and physical factors involved in chronic idiopathic prostatitis. J Psychosomatic Research, 41: 313-325, 1996.
__________________________
Received: September 30, 1999
Accepted: October 5, 1999
_______________________
Correspondence address:
Anthony J. Schaeffer, M.D.
Dept. of Urology, Univ. Northwestern
Tarry Building 11-715
303 East Chicago Avenue
Chicago, Illinois, USA, 60611-3008
Fax: ++ (1)(312) 908-7275
E-mail: ajschaeffer@nwu.edu