PRENATAL
INTERVENTION FOR URINARY OBSTRUCTION AND MYELOMENINGOCELE
(
Download pdf )
HUBERT S. SWANA, RONALD S. SUTHERLAND, LAURENCE BASKIN
Department of Urology, University of California San Francisco, and Department of Surgery, Tripler Army Medical Center, San Francisco, California, USA
ABSTRACT
Widespread use of ultrasonography has resulted in an increase in the recognition of fetal hydronephrosis. The enthusiasm that accompanied early interventions has been tempered by the experience and results obtained over the past 2 decades. The goal has remained the same: to identify patients with serious prenatal obstruction and to identify those which may benefit from intervention. Myelomeningocele remains a devastating congenital anomaly. Fetal and experimental studies suggested that patients with myelomeningocele could benefit from prenatal intervention. Advances in technology and perinatal management have made intervention for more complex malformations such as myelomeningocele possible. This article will review current knowledge and will detail rational management for the management of prenatal hydronephrosis. The current state of antenatal myelomeningocele repair and the urologic implications will be described as well.
Key
words: fetus; congenital abnormalities; prenatal diagnosis; myelomeningocele;
intrauterine; surgery; fetoscopy
Int Braz J Urol. 2004; 30: 40-8
INTRODUCTION
During
the past 2 decades pediatric urologists have begun acquiring patients
with antenatally detected conditions. With the widespread use of maternal
ultrasound, fetal hydronephrosis has become increasingly detected, and
it comprises the most common prenatally diagnosed malformation. The concept
of the unborn child as a potential surgical patient has become firmly
established (1). Fetal medicine has rapidly evolved since early experiences
with the management of fetal hydronephrosis. With time, the natural history
and pathophysiology of urinary tract obstruction has become better understood.
Improvements in diagnostic imaging tools, advances in fetal urine sampling,
enhanced interventional techniques and equipment, and a better understanding
of the risks and outcomes in these babies have helped to develop rational
intervention and observation strategies. Nevertheless, the management
of the fetus with hydronephrosis has remained controversial.
The purpose of this article is to review
antenatal intervention and its history. The basics of normal fetal development
will be integrated with the techniques used to diagnose disorders of the
urinary tract. Particular attention will be devoted to the diagnostic
techniques of ultrasound, fetal urine sampling and amniocentesis. Newer
modalities such as fetal magnetic resonance imaging (MRI) will be described.
Intervention for patients with myelomeningocele and the implications for
urinary tract function will be discussed. Methods of intervention will
be described along with their indications, contraindications, and complications.
SPECTRUM OF ANTENATAL DISORDERS: EMBRYOLOGY AND PATHOPHYSIOLOGY
Perturbation
of the developing ureteral bud and its intended target, the metanephric
blastema by distal obstruction affects normal renal development (2-7).
By the 5th week of gestation, the ureteral bud rises from the mesonephric
duct. It then begins to lengthen and canalize. Induction of the metanephric
blastema occurs by the end of week 7. Primitive renal function begins
between week 7 and 9, and by week 20, about 1/3 of the total number of
nephrons are present. Nephrogenesis is complete by the 32nd week of fetal
life, after which no demonstrable increase in the number of glomeruli
is noted (8-10).
The spectrum of deleterious changes seen
in antenatal urinary obstruction is the result of multiple factors. They
include the time of onset, duration, and degree of urinary obstruction.
In general the earlier the obstruction occurs the more disturbed the development
of the fetal kidney (8). Renal dysplasia, the most severe form of renal
injury and maldevelopment, has been attributed to a very early effect
of elevated pressures in the urinary (2) and alternatively by ureteral
bud malposition with subsequent misconnection between the bud and the
metanephric blastema (4,11). Without ureteral bud induction, the blastema
fails to develop. One sees clusters of disorganized metanephric structures
surrounded by abundant fibrous tissue. Ninety per cent of cases of renal
dysplasia are associated with urinary obstruction during nephrogenesis.
Sonography is highly specific for diagnosing dysplasia and the demonstration
of renal cysts in a fetus with known obstructive uropathy effectively
indicates the presence of dysplasia (12). The absence of cortical cysts,
however, does not exclude renal dysplasia.
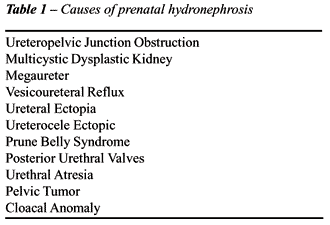
Dilation of the urinary tract can be due
to, ureteropelvic junction obstruction (UPJO), congenital obstructed and
nonobstructed megaureter, multicystic kidney, duplication anomalies with
upper pole ectopia or obstructing ureterocele, and vesicoureteral reflux
(VUR) (13). Obstruction of the upper urinary tract from physiologic ureterectasis
or from UPJO is rarely complete. One must also exclude physiological hydronephrosis
which usually spontaneously resolves prior to delivery or within the first
year of life (14) (Table-1).
More distal causes of obstruction include
posterior urethral valves, urethral atresia, cloacal anomalies, and prolapsing,
obstructing ureteroceles. These entities can result in marked distortion
of both ureters and kidneys as well as pathological bladder changes. The
prune belly syndrome is rarely been associated with renal obstruction
even though the urinary tract is massively dilated. Some have argued that
the characteristic urinary tract dilation is a consequence of transient
fetal urethral obstruction (15).
Spinal cord and subsequent vertebral formation
begins at day 18 of gestation. Neural tube in-folding (neurulation) occurs
between 18 and 27 days of gestation and is normally followed by migration
of mesodermal tissue around the developing spinal cord. The mesoderm gives
rise to the vertebral arches, as well as the spinal and back musculature.
The location, timing and extent of the abnormal closure lead to the varying
degrees and levels of neural tube defects. Lesions can vary to include
spina bifida occulta, (a closed tube defect), meningocele, (a protruding
meningeal sac without neural elements), myelomeningocele (a menigeal sac
with neural elements) and lipomeningocele (a meningeal sac with neural
elements and fatty tissue). Myelomeningocele is the most common neural
tube defect. Lumbar vertebrae are most commonly involved followed by sacral,
thoracic and cervical vertebrae in decreasing frequency. Failure to close
at the caudal end results in a distal defect with resultant lower limb
paralysis and bladder dysfunction (16). An Arnold-Chiari type II malformation
occurs in up to 85% of children with myelomeningocele (MMC). There can
be herniation of the cerebellar tonsils through the foramen magnum. This
can result in obstruction of the fourth ventricle and necessitates ventriculoperitoneal
shunting.
Urology morbidity in patients with MMC is
significant. Myelodysplasia can result in a poorly compliant bladder,
sphincteric dysfunction, secondary vesicoureteral reflux, a predisposition
to urinary tract infections, possible renal scarring and renal failure
(17). Urologic morbidity is the sequela of neurologic injury. The neurologic
deficit seen in MMC is believed to be due to several factors. The first
is defective development. Evidence supporting a secondary insult to the
exposed spinal cord has resulted in a “two hit hypothesis”.
Histologic findings support the idea that the exposed spinal cord is vulnerable
to damage by physical trauma as the cord contacts the uterine wall. Physical
trauma, and the toxic effects of amniotic fluid and meconium to the exposed
spinal cord have been reported (18-20). Fetal lower limb movements have
been described in fetuses with MMC at 16-17 weeks (21). Animal studies,
in which laminectomy was performed at mid-gestation, compared in-utero
repair to no treatment. The animals that underwent fetal intervention
were spared flaccid paralysis and incontinence of urine and stool (22).
Histologic specimens of bladder tissue from children with spina bifida
reveal increased intracellular matrix between muscle bundles, decrease
muscarinic receptor density abnormal smooth muscle growth, and decreased
innervation (23-25). These factors likely contribute to bladder dysfunction
in human spina bifida patients.
DIAGNOSIS OF OBSTRUCTION
Ultrasound
The evolution of fetal intervention has
paralleled the advancements in ultrasound technology. High resolution,
real time imaging and the ability to choose focal zone depth have been
major advances in ultrasonography (26). Fetal positioning plays a critical
role in the interpretation and understanding of the fetal anatomy. The
prone fetus is in the optimal position for imaging the kidneys (1). While
the kidneys can be seen as early as the 15th week reliable imaging is
not possible until week 18 (26).
Hydronephrosis is the most common cause
of an abdominal mass in the neonate, and antenatal sonography readily
detects fetal urinary tract dilation (1). Pelviectasis is found in 18%
of normal fetuses (27). Both caliectasis and an anteroposterior pelvic
diameter of greater than 10 mm have been proven to be reliable predictor
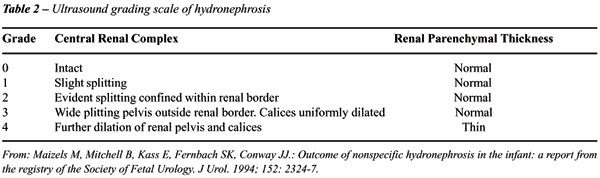
of fetuses in need of postnatal urologic evaluation (28,29). The Society
of Fetal Urology has adopted a grading system form hydronephrosis, which
is widely used by pediatric urologists today (Table-2).
Prenatal sonography is very sensitive in
differentiating ureteropelvic junction obstruction from other causes of
obstruction and dilation (30,31).
While ultrasonography remains the primary
imaging modality for the screening and evaluation of congenital abnormalities,
it is not without limitations. Maternal obesity, oligohydramnios and suboptimal
fetal position can make accurate imaging difficult. Early use of MRI was
limited by slow acquisition times and was hampered by fetal motion. Newer
methods have been developed that can reduce acquisition times and provide
excellent image quality without the need for fetal sedation or paralysis
(32). MRI can provide images unaffected by fetal position, maternal obesity,
oligohydramnics, or overlying bowel and possibly could provide a definitive
diagnosis of obstructive uropathy (33,34).
MRI seems to be superior in identifying
the intracranial lesions such as agenesis of the corpus callosum, cerebellar
dysplasia and holoprosencephaly that can accompany myelomeningocele (35).
In the future, clinical decisions may be based on analysis of chemical
and molecular events with MRI (36). Presently, MRI is a useful adjunct
to ultrasonography. MRI provides additional information in myelomeningocele,
other complex fetal cases, and cases of hydronephrosis with indeterminate
US studies.
Fetal Urine
and Amniotic Fluid Testing
Invasive acquisition of fetal urine for
analysis has become one of the most important measures of assessing fetal
renal function. Measurement of fetal urine electrolytes and urinary proteins
is a useful guide to the clinician in deciding whether prenatal intervention
is indicated. Additional methods of assessing the overall status of the
fetus include amniocentesis, chorionic villus sampling, percutaneous umbilical
blood sampling (all for karyotyping), as well as amniotic fluid volume
and its biochemical constituent measurement.
Fetal urine is normally hypotonic reflecting
developing glomerular and tubular function (37). The amniotic fluid, in
comparison, is somewhat hypertonic, and is not as reliable an index of
renal function as the fetal urine. Determination of human fetal renal
function is limited to simple concentration of specific urinary constituents.
More physiologic measurements of glomerular function, while possible,
are not routinely performed (38). Clearance of iothalamate has been done
and shown to be non-predictive of renal outcome (10). Retrospective analysis
of individual urine constituents have shown that a sodium of less than
100 mEq/L, osmolality less than 210 mOsm/L and chloride less than 90 mEq/L,
if accompanied by lack of ultrasonographic evidence of dysplasia, are
helpful in predicting residual fetal renal function. By categorizing patients
according prognosis, assessment of the potential efficacy of intervention
ca be made (39). Elder et al. (39) and Johnson et al. (40) separately
suggested that single determinations of urinary electrolytes may not be
useful. Johnson et al. (41) proposed providing transient relief of obstruction
by vesicocentesis followed by sequential sampling (3 or 4 samples over
several days) of urinary electrolytes. This was felt to provide an assessment
of the severity of the renal injury and potential for reversibility of
renal injury. Those fetuses that experience an improvement in their biochemical
parameters following decompression may benefit most from interventional
therapy (41). Others have suggested that sampling of fetal urine electrolytes
and osmolarity is not an optimal method to evaluate fetal renal function
and recommend continued search for a better substance (42-44). A serum
marker, which has provided some clinical utility, is beta-2 microglobilin.
It is excreted by the kidney without placental cross-over so that fetal
levels represent fetal renal function. One can see an elevation in renal
dysplasia (45). Other urinary constituents commonly associated with the
presence of renal disease include proteins such as albumin, retinol binding
protein, and N-acetyl-b-glucosaminidase have been studied (45). Unfortunately
human fetal urine sampling lacks known control normals at different stages
of development. Further limitation includes the inability to accurately
and physiologically measure renal function by fractional excretion of
biochemical constituents and glomerular filtration without risky invasive
fetal and maternal testing (46).
INTERVENTIONAL TECHNIQUE
Current
Indications and Contraindications
For most fetuses with obstructive uropathy,
intervention is not necessary (47) (Figure-1). The selection criteria
for fetal therapy of obstruction evolved such that patient selection is
presently good enough to avoid intervention in patients who are either
too well (no benefit) or too ill to recover (48). It has been conclusively
shown that decompression in utero will restore amniotic fluid, which can
prevent the development of fatal pulmonary hypoplasia. What seems less
clear is whether or not in utero decompression can arrest or reverse cystic
dysplastic changes caused by obstruction (48).
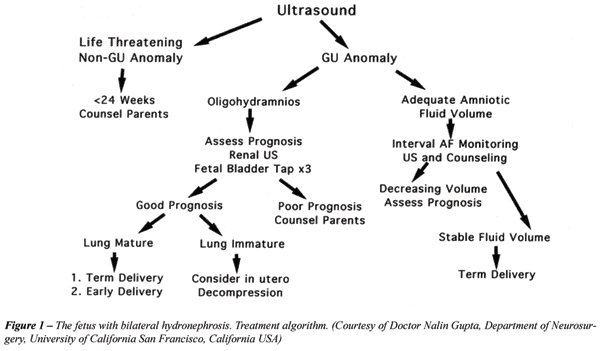
Spontaneous resolution of hydronephrosis
is common, which has led to a more cautious approach to fetal intervention
(1,49). In most cases with normal amniotic fluid volume, the mother should
be followed by serial ultrasound examinations, and the fetus should be
evaluated and treated postnatally. If moderate to severe oligohydramnios
develops, the fetus should undergo complete prognostic evaluation to assess
the potential for normal renal and pulmonary function at birth. If the
ultrasound demonstrates presence of dysplasia, aggressive obstetrical
care or prenatal decompression is not indicated. When preserved renal
function is predicted, early delivery for postnatal decompression is indicated
if the lungs are mature. Early delivery usually does not compromise pulmonary
function as long as amniotic fluid volume has been maintained (1). If
the lungs are immature, however, in-utero decompression can be considered.
METHODS OF INTERVENTION
Urinary
Tract
Early attempts at bladder decompression
in the late 70’s and early 80’s attempted a Seldinger-type
procedure, but with limited success. A tight fitting double pigtail catheter
placed over a puncture needle using a pusher worked; although it was far
from ideal. Due to the difficulties in catheter placement, migration and
plugging, Malecot-type and external coil type catheters were developed.
Open fetal surgery began in the early 1980’s,
and was performed on eight highly selected cases of obstructive uropathy
from 18-24 weeks gestation. Unfortunately this method of treatment carried
significant morbidity predominantly from preterm labor (47,48,50). As
a result, open fetal surgery to correct urinary tract obstruction has
not since been performed. In those early patients, open decompression
procedures included cutaneous vesicostomy in 7 and bilateral ureterostomies
in 1. Only 4 had prolonged return of normal amniotic fluid and had adequate
pulmonary function at birth. Of these only two have normal renal function
at ages 5 and 8 years (51).
With advances in endoscopic equipment, the
technique of transuterine endoscopy was developed at the University of
California, San Francisco (52). MacMahon and associates reported a similar
fetoscopic approach in a human fetus with prune belly syndrome and oligohydramnios
at 17 + weeks. They used a Neodymium-Yag laser to create a vesicoamniotic
shunt, which was successful at restoration of the amniotic fluid volume.
The fistula closed by 33 weeks and the child was delivered early with
normally developed lungs (53). Fetal cystocopy and valve ablation has
been reported. Both antegrade and retrograde techniques have been reported.
Flexible and rigid instruments were used as well. Significant fetal mortality
was reported (54).
Myelomeningocele
Repair of MMC has been attempted both endoscopically
and through open surgery via a hysterotomy. While technically possible,
surgery for MMC is not presently being performed via a fetoscopic approach.
Fetoscopy is limited by the need for multiple port sites, which can lead
to membrane fixation and rupture as the uterus enlarges. In addition it
is difficulty to visualize large spinal defects and requires prolonged
operative times (55).
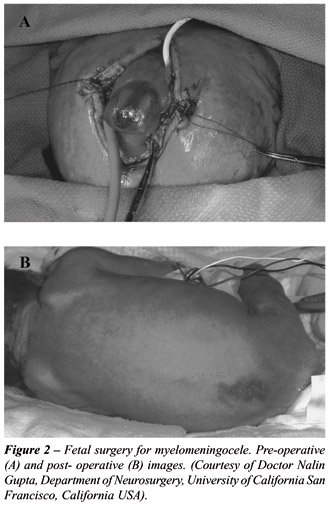
Open fetal surgery requires careful planning
(Figure-2). Attempts at enhancing fetal lung maturity are made through
the use of preoperative glucocoritosteroid administration to the mother.
Broad spectrum antibiotics and balanced anesthesia allow the procedure
to take place. The amniotic fluid is removed and kept in sterile warm
syringes. A standard neurosurgical closure is performed through an approximately
8 cm hysterotomy. The neural placode is dissected from the adjacent arachnoid
tissue and placed in the spinal canal. The dura is then dissected off
for another layer of coverage. The skin then is freed for a final layer
of closure. The amniotic fluid and added antibiotics are replaced and
the uterus closed. Phophylactic tocolytics are then used (56).
RESULTS
AND COMPLICATIONS
Urinary
Tract
Early results of prenatal bladder shunting
reassured physicians that the procedures could be performed safely and
that catheter drainage was well tolerated in most cases. Whether prenatal
shunting improves outcome remains a different matter. Patient selection
is critical. One must find a dilated urinary tract with severe enough
obstruction to compromise renal and pulmonary function at birth, and yet
not so severe that renal function cannot be salvaged with decompression
(1).
Reviews by Coplen, McLorie and Baskin have
shown several things. First, obstruction and dysplasia are difficult to
predict. Second, while technically feasible, fetal interventions were
associated with only a 47% survival rate and a 45% of fetuses had complications
(39,56-58). Third, even though oligohydramnios could be reversed, the
ability to sustain good renal function was variable. Lastly, specific
prenatal parameters that were effective in predicting good renal function
have note been found (52,58).
The most common complication arising from
open in utero fetal intervention is the instigation of preterm labor (50).
Catheters can fail either by plugging. Incorrect placement despite ultrasound
guidance has resulted in fetal injury, and death (59). Reinsertions increase
the risks of fetal injury and infections. Chorioamnionitis can sometimes
resulting in pregnancy termination (51).
Myelomeningocele
Prenatal surgery for myelomeningocele has
yielded some unexpected outcomes. Tubbs et al. were not able to show improved
lower extremity function in patients that underwent intrauterine intervention
(59).
Bruner et al. compared 29 fetal surgery
patients with 23 controls matched for level of defect, diagnosis, calendar
time and practice parameters (60). They reported a statistically significant
(P = 0.01) decrease in the need for ventriculoperitonel shunt placement
and a lower incidence of hindbrain herniation (P = 0.001). Patients who
underwent fetal surgery did however have a higher risk of oligohydramnios
(48% vs. 4%; P = 0.001), and admissions for preterm contractions (50%
vs. 9%; P = 0.002). They were also more susceptible to prematurity. Age
at delivery was earlier (33 vs. 37 weeks; P < 0.001) and birth weight
were lower (2171 vs. 3075 gm; P < 0.001) (56).
The effect of fetal intervention for myelomeningocele
on postnatal bladder function has been studied (57,58). Despite the early
repair, patterns of abnormal bladder function were exhibited. One still
sees poor compliance, poor detrusor contractility, detrusor-sphincter
dysynergia, hydronephrosis and vesicoureteral reflux. The previously described
global defect in bladder development makes success of fetal surgery to
preserve or improve bladder function unlikely. Additional studies are
ongoing.
CONCLUSIONS
The field of fetal medicine has grown over the past two decades. Well-defined animal studies have yielded clues to the natural history and pathogenesis of obstructive uropathy and the efficacy of interventional techniques to ameliorate the sequelae of such obstruction. With advances in technology, the complexity of anomalies, which can be treated, has increased, as evidenced by the growing experience with fetal myelomeningocele repair. In addition these new scenarios provide new ethical challenges. Carrying out procedures in human fetuses must continue to be appropriately cautious and circumspect. The uncertainties and true pathologic processes surrounding urinary tract obstruction must continue to be explored. More reliable methods of determining fetal renal function lay on the horizon. Interventional techniques continue to evolve and improve. Because of the potential risks for preterm labor and maternal compromise, fetal surgery should continue to be performed only for carefully selected cases at centers that are equipped with a multidisciplinary health care team committed to ongoing, well-designed research protocols.
REFERENCES
- Harrison M, Filly R: The Unborn Patient, 2nd ed. Philadelphia, W.B. Saunders Co. 1991.
- Beck AD: The effect of intra-uterine urinary obstruction upon the development of the fetal kidney. J Urol. 1971; 105: 784-9.
- Berman DJ, Maizels M: The role of urinary obstruction in the genesis of renal dysplasia. J Urol. 1983; 128: 1091-6.
- Barrett DM, Wineland RE: Renal cell carcinoma in multicystic dysplastic kidney. Urology. 1980; 15: 152-4.
- Longino LA, Martin LW: Abdominal masses in the newborn infant. Pediatrics. 1958; 21: 596-604.
- Glick PL, Harrison MR, Noall RA, Villa RL: Correction of congenial hydronephrosis in utero III. Early mid-trimester ureteral obstruction produces renal dysplasia. J Pediatr Surg. 1983; 18: 681-7.
- Bellinger MF, Comstock CH, Grosso D, Zaino R: Fetal posterior urethral valves and renal dysplasia at 15 weeks gestational age. J Urol. 1983; 129: 1238-9.
- Gasser B, Mauss Y, Ghnassia JP, Favre R, Kohler M, Yu O, et al.: A quantitative study of normal nephrogenesis in the human fetus: its implication in the natural history of kidney changes due to low obstructive uropathies. Fetal Diagn Ther. 1993; 8: 371-84.
- Potter E: Normal and Abnormal Development of the Kidney. Yearbook Medical. 1972.
- Glick PL, Harrison MR, Golbus MS, Adzick NS, Filly RA, Callen PW: Management of the fetus with congenital hydronephrosis II: Prognostic criteria selection for treatment. J Pediatr Surg. 1985; 20: 376-87.
- King LR: The Management of Multicystic Kidney and Ureteropelvic Junction Obstruction. In King L (ed.), Urologic Surgery in Neonates and Young Infants. Philadelphia, W.B. Saunders Co. 1988; p. 140.
- Mahony HS, Filly RA, Callen PW, Hricak H, Gobbus MS, Harrison MR, et al.: Fetal renal dysplasia: sonographic evaluation. Radiology. 1984; 152: 143-6.
- Gordon AC, Thomas DFM, Arthur RJ, Irwing HC, Smith SE: Prenatally diagnosed reflux: a follow-up study. Br J Urol. 1990; 65: 407-12.
- Anderson PAM, Rickwood AMK: Featurers of primary vesicoureteric reflux detected by prenatal sonography. Br J Urol. 1991; 67: 267.
- Snow BW, Duckett JW: Prune Belly Syndrome. In: Gillenwater JY, Grayhack JT, Howards S, Duckett JW (eds.), Adult and Pediatric Urology, St Louis, Mosby. 1991; 2nd ed., p. 1921.
- Moore KL, Persaud TVN: The Developing Human: Clinically Oriented Embryology. Philadelphia, W.B. Saunders Co. 2003; pp. 428-33.
- Muller T, Arbeiter K, Aufricht C: Renal function in myelomeningocele: risck factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol. 2002; 12: 479-84.
- Meuli M, Meuli-Simmeti C, Hutchins GM, Seller MJ, Harrison MR, Adzick NS: The spinal cord lesion in human fetuses with myelomeningocele: Implications for feta surgery. J Ped Surg. 1997; 32: 448-52.
- Drewek MJ, Bruner JP, Whetsell WO, Tulipan N: Quantitative analysis of the toxicity of human amniotic fluid to cultured rat spinal cord. Ped Neurosurg. 1997; 27: 190-3.
- Correia-Pinto J, Reis JL, Hutchins GM, Baptista MJ, Estevão-Costa J, Flake AW, et al.: In utero meconium exposure increases spinal cord necrosis in a rat model of myelomeningocele. J Ped Surg. 2002; 37: 488-92.
- Korenromp MJ, van Good JD, Bruinese HW, Kriek R: Early fetal movements in myelomeningocele. Lancet. 1986; 8: 917-8.
- Meuli M, Meuli-Simmen, Hutchins GM, Yingling CD, Hoffman KM, Harrison MR, et al.: In utero surgery rescues neurologic function at birth in sheep with spina bifida. Nat Med. 1995; 1: 342-7.
- Shapiro E, Becich MJ, Perlman E, Lepor H: Bladder wall abnormalities in myelodysplastic children: a computer assisted morphometric analysis. J Urol. 1991; 145: 1024-9.
- Gup DI, Baumann M, Lepor H, Shapiro E: Muscarinic cholinergic receptors in normal pediatric and myelodysplastic bladders. J Urol. 1989; 142: 595-9.
- Shapiro E, Seller MF, Lepor H, Kalousek DK, Hutchins GM, Perlman EJ, et al.: Altered smooth muscle development in the lower genitourinary and gastrointestinal tract of the male fetus with myelomeningocele. J Urol. 1998; 160: 1047-53.
- Merguuerian P: The evaluation of prenatally detected hydronephrosis. Mongraph Urol. 1995; 16: 1-5.
- Hoddick WK, Filly RA, Mahony BS, Callen PW: Minimal fetal renal pyelectasis. J Ultrasound Med. 1985; 4: 85-9.
- Cendron M, Morin L, Crombleholme T: Early minimal fetal hydronephrosis: clinical outcomes and implications for management. American Academy of Pediatrics. Pediatric Urology Sections (Dallas, Texas). 1994; Abstract # 30.
- Birken G, Vane D, King Dea: Adenocarcinoma arising in a multicystic dysplastic kidney. Pediatr Surg. 1985; 20: 619.
- Manning FA, Harrison MR, Rodeck C: Catheter shunts for fetal hydronephrosis and hydrocephalus. N Engl J Med. 1986; 315: 336-40.
- Yamashita Y, Namimoto T, Abe Y, Takahashi M, Iwamasa J, Mujazaki K, et al.: MR imaging of the fetus by a HASTE séquense. AJR. 1997; 168: 513-9.
- Coakley FV, Hriak H, Filly RA, Barkovich AJ, Harrison MR: Complex fetal disorders: effect of MR imaging on management-preliminary clinical experience. Radiology. 1999; 213: 691-6.
- Aaronson OS, Hernanz-Schulman M, Bruner JP, Reed GW, Tulipan NB: Myelomeningocele: Preneatal evaluation – Comparison between transabdominal US and MR imaging. Radiology. 2003; 227: 839-43.
- Levine D, Barnes PD, Madsen JR, Abbott J, Mehta T, Edelman RR: Central nervous system abnormalities assessed with prenatal magnetic resonance imaging. Obstet Gynecol. 1999; 94: 1011-9.
- Tempany CMC, McNeil BJ: Advances in biomedical imaging. JAMA. 2001; 285: 562-7.
- Woodard Jr: Neonatal and perinatal emergencies. In: Harrison JH, Gittes RF, Perlmutter AD, et al. (eds.): Campbell’s Urology, Philadelphia, W.B. Saunders Co. 1979, 4th ed., p. 1855.
- Adzick NS, Sutton LN, Crombelhome TM, Flake AW: Successful fetal surgery for spina bifida. Lancet. 1998; 852: 1675-6.
- Crombleholme TM, Harrison MR, Golbus MS, Longaker MT, Langer JC, Callen PW, et al.: Fetal intervention in obstructive uropathy: prognostic indicators and efficacy of intervention. Am J Obstet Gynecol. 1990;162:1239-44.
- Elder JS, O’Grady JP, Ashmead G, Duckett JW, Philipson E: Evaluation of fetal renal function: unreliability of fetal urinary electrolytes. J Urol. 1990; 144: 574-8.
- Johnson MP, Corsi P, Bradfield W, Hume RJ, Smith C, Flake AW, et al.: Sequential analysis improves evaluation of fetal renal function in obstructive uropathy. Am J Obstet Gynecol. 1995; 173: 59-65.
- Johnson MP, Bukowski TP, Reitleman C, Isada NB, Pryde PG, Evans ML: In utero surgical treatment of fetal obstructive uropathy: a new comprehensive approach to identify appropriate candidates for vesicoamniotic shunt therapy. Am J Obstet Gynecol. 1994; 170: 1770-6.
- Elder JS, Duckett JW, Snyder HM: Intervention for fetal obstructive uropathy: has it been effective? The Lancet. 1987; Oct 31, 8566: 1007-10.
- Wilkins IA, Chitkara U, Lynch L, Goldberg JD, Mehalek KE, Berkowitz RL: The nonpredictive value of fetal urinary electrolytes: Preliminary report of outcomes and correlations with pathologic diagnosis. Am J Obstet Gynecol. 1987; 157: 694-8.
- Mandell J, Blyth BR, Peters CA, Retik AB, Estroff JA, Benacerraf BR: Structural genitourinary defects detected in utero. Radiology. 1991; 178: 193-6.
- Dommergues M, Muller F, Ngo S, Hohlfeld P, Oury JF, Bidat L, et al.: Fetal serum beta-2-microglobulin predicts postnatal renal function in bilateral uropathies. Kid Int. 2000; 58: 312-6.
- Longaker MT, Golbus MD, Filly RA, Rosen MA, Chang SW, Harrison MR: Maternal outcome after open fetal surgery. JAMA. 1991; 265: 737-41.
- Adzick NS, Harrison MR: The unborn surgical patient. Curr Probl Surg. 1994; 31: 1-68.
- Onen A, Jayanthi VR, Koff SA: Long term follow-up of prenatally detected severe bilateral newborn hydronephrosis initially managed nonoperatively. J Urol. 2002; 168: 1118-20.
- Harrison MR, Adzick NS: The fetus as a patient. Surgical considerations. Ann Surg. 1991; 213: 279-91.
- Crombleholme TM, Harrison MR, Golbus MS, Longaker MT, Langer JC, Callen PW, et al.: Fetal intervention in obstructive uropathy: prognostic indicators and efficacy of intervention. Am J Obstet Gynecol. 1990; 162: 1239-44.
- Estes JM, MacGillivray TE, Hedrick MH, Adzick NS, Harrison MR: Fetoscopic surgery for the treatment of congenital anomalies. J Pediatr Surg. 1993; 27: 950-4.
- Najmaldin A, Burge DM, Atwell JD: Fetal vesicoureteric reflux. Br J Urol. 1990; 65: 403-6.
- Quintero RA, Hume R, Smith C, Johnson MP, Cotton DB, Romero R, et al.: Percutaneous fetal cystoscopy and endoscopic fulgaration of posterior urethral valves. Am J Obstyet Gynecol. 1995: 172: 206-9.
- Olutoye OO, Adzick NS: Fetal surgery for myelomeningocele. Semin Perinatol. 1999; 23: 462-73.
- Coplen DE: Prenatal intervention for hydronephrosis. J Urol. 1997; 157: 2270-7.
- McLorie G, Farhat W, Khoury A, Geary D, Ryan G: Outcome analysis of vesicoamniotic shunting in a comprehensive population. J Urol. 2001; 166: 1036-40.
- Holmes N, Harrison MR, Baskin LS: Fetal surgery for posterior urethral valves: Long-term postnatal outcomes. Pediatrics. 2001; 108: E7.
- Harrison MR, Golbus MS, Filly RA, Anderson RL, Flake AW, Rosen M, et al.: Feta hydronephrosis: selection and surgical repair. J Pediatr Surg. 1987; 22: 556-8.
- Tubbs RS, Chambers MR, Smyth MD, Bartolucci AA, Bruner JP, Tulipan N, et al.: Late gestational myelomeningocele repair does not improve lower extremity function. Ped Neuro Surg. 2003; 38: 128-32.
- Bruner JP, Tulipan N, Paschall RL, Boehm FH, Walsh WF, Silva SR, et al.: Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999; 282: 1819-25.
_______________________
Received: October 13, 2003
Accepted: November 17, 2003
_____________________
Correspondence address:
Dr. Laurence S. Baskin
Department of Urology
UCSF, Children’s Medical Center
400 Parnassus Ave., Rm. 610
San Francisco, California, 94143-0330, USA
Fax: + 1 415 476-8849
E-mail: lbaskin@urol.ucsf.edu