COMPARISON
OF THE CLINICAL AND PATHOLOGIC STAGING IN PATIENTS UNDERGOING RADICAL
CYSTECTOMY FOR BLADDER CANCER
(
Download pdf )
SEAN MCLAUGHLIN, JON SHEPHARD, ERIC WALLEN, SUSAN MAYGARDEN, CULLEY C. CARSON, RAJ S. PRUTHI
Division of Urologic Surgery, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
ABSTRACT
Purpose: Radical cystectomy (RCx) is perhaps the most effective therapeutic
approach for patients with muscle-invasive bladder cancer. Unfortunately,
clinical staging is imprecise and the degree of understaging remains high.
This study retrospectively evaluated patients undergoing RCx with regard
to pathologic outcomes and degree of upstaging to better identify features
that may lessen clinical understaging.
Materials and Methods: 141 consecutive patients
with urothelial bladder carcinoma who were candidates for RCx with curative
intent were retrospectively evaluated. Preoperative clinical and pathological
(i.e. TURBT) features were compared to pathological outcomes in the cystectomy
specimen. Patients were also evaluated as to whether cystectomy was performed
as their primary (n = 91) versus secondary (n = 50) treatment for recurrent/progressive
disease. Date of cystectomy (≤ 5 years vs. > 5 years prior to
study) was also analyzed.
Results: Of the 141 patients, 54% were upstaged
on operative pathology. The greatest degree of upstaging occurred in those
with invasive disease preoperatively (cT2-T3). Twenty-six percent of all
patients had node-positive disease, and 75% of cT3 patients were node-positive.
Seven of 101 (7%) patients with clinical T2 disease were unresectable
at the time of surgery. In the primary (vs. secondary) RCx group, more
patients were upstaged (63% vs. 40%), non-organ confined (62% vs. 38%),
and LN positive (31% vs. 20%). In the more modern cohort, the degree of
upstaging was not improved.
Conclusions: Pathologic findings after RCx often
do not correlate with preoperative staging. Over half of patients undergoing
cystectomy are upstaged on their operative pathology. An improved understanding
of the relative frequency of upstaging in cystectomy patients may have
important implications in the decision-making and selection for neoadjuvant
and adjuvant therapies for these high-risk populations.
Key
words: bladder cancer; neoplasm staging; cystectomy; pathology
Int Braz J Urol. 2007; 33: 25-32
INTRODUCTION
After
prostate cancer, bladder cancer is the most common urologic and the fifth
most common overall malignancy.
In 2005, there were approximately 63,000
new cases of bladder cancer diagnosed and over 13,000 disease-related
deaths in the United States (1). The majority of new bladder tumors are
superficial (60 - 75%) and of those, up to 20% can be expected to progress
to muscle invasive disease (2). Nevertheless, a significant number of
muscle-invasive tumors are diagnosed at initial presentation in patients
with no prior history of TCCa.
The most common therapeutic approach for
invasive bladder cancer is radical cystectomy. Recent improvements in
surgical technique and perioperative management have reduced complication
rates and operative mortality for this procedure (3,4). Despite the improvements
in surgical morbidity, up to 50% of patients undergoing cystectomy will
experience local or distant recurrence. Unfortunately, most of these patients
who are destined to recur are not easily distinguished upon pre-operative
evaluation. Such findings highlight the significant clinical under-staging
that occurs in bladder cancer patients undergoing cystectomy. Our inability
to prospectively identify non-organ-confined disease or systemic micrometastases
remains a shortcoming of current preoperative evaluation. Consequently,
many patients are upstaged at the time of surgical exploration and extirpation.
This retrospective review sought to characterize
patients who are upstaged at the time of cystectomy in order to better
identify pre-operative factors, which may contribute to more aggressive/advanced
disease. Factors including cross-sectional imaging (and improvements that
may have occurred in recent years), impact of bladder-sparing therapies
(i.e. those undergoing a primary versus secondary cystectomy), as well
as other clinical and demographic factors were also evaluated.
MATERIALS AND METHODS
We
retrospectively examined the medical records of 141 consecutive patients
with urothelial cancer of the bladder that underwent radical cystectomy
(RCx) for clinically-localized disease and with curative intent from 1990
to 2002. Age, race, gender, tumor grade, clinical stage, pathological
stage information for each patient were extracted.
Patients were also analyzed as to whether
RCx was performed as primary therapy after their initial diagnosis (primary
cystectomy or PRCx) (n = 91), or whether RCx was performed for recurrent
or progressive disease after bladder-sparing treatments were first utilized
(secondary cystectomy or SRCx) (n = 50). Of note, PRCx patients had no
prior history of intravesical therapies and received no neoadjuvant treatment
modalities (e.g. chemotherapy or radiation therapy). In the SRCx group,
all patients had received bladder-sparing regimens after their initial
diagnosis including intravesical BCG immunotherapy (n = 41), intravesical
chemotherapy (n = 8), partial cystectomy (n = 3), radiation therapy (n
= 2), or a combination of these modalities.
In addition, comparisons were made with
regard to date of cystectomy (before 1997, n = 54 vs. 1997-2002, n = 87)
in order to evaluate for possible improvements in clinical staging that
may have occurred in the most recent 5 years (e.g. improvements in resolution
of cross-sectional imaging).
All cystectomy specimens were received fresh
in pathology, opened anteriorly, pinned open and fixed overnight in 10%
buffered formalin. The next day, the external aspect of the bladder was
inked, and margin sections were taken of the ureters and urethral margins.
Standard bladder sections were taken to include 3-4 samples of any grossly
visible tumor to include the areas of deepest gross invasion, sections
of any mucosal abnormality, and random sections of the dome, anterior,
posterior, right lateral and left lateral wall, and trigone. Sections
of any attached organs were taken if present. In male patients, two sections
of each lobe of the prostate and one section from right and left seminal
vesicles were taken as initial sampling, with additional sections submitted
if significant findings were present on these initial sections. One section
of each submitted lymph node was taken. All sections were routinely processed,
paraffin embedded, and stained with hematoxylin and eosin.
The nonparametric Jonkheere-Terpstra method
was used to test for ordered differences among categories. With this test,
the null hypothesis is that the distribution of the response does not
differ across ordered categories. The nonparametric Wilcoxon signed-rank
test was used on calculated pair difference values. All p values were
adjusted using the Bonferroni method to account for multiple testing or
comparisons. Statistical analyses were performed with SAS statistical
software, Version 8.2, SAS Institute Inc., Cary, NC.
RESULTS
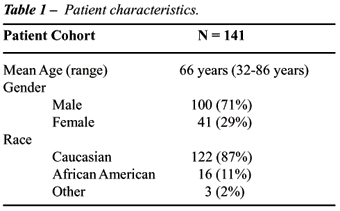
The
clinical characteristics of the patient cohort are shown in Table-1. Of
note, there were no differences in age, gender composition, or racial
composition between those upstaged versus those who were not. It should
be noted that only 4 patients (2.8%) were classified as low grade or grade-1:
all others were classified as high grade or grade-2 or 3. Since the vast
majority were classified as moderate or high grade, grade was not therefore
not a useful distinguishing or predictive characteristic with regarding
to upstaging of disease.
Of 141 patients, 54% were upstaged at pathologic
staging. The degree of upstaging stratified by clinical stage is shown
in Table-2. The greatest degree of upstaging occurred in those with invasive
disease pre-operatively (cT2-T3). Of note, 26% of all patients were ultimately
proven to have node-positive disease, and 75% of those with clinical T3
disease were node-positive. In addition, 7 of the 101 (7%) patients with
clinical T2 disease were found to be unresectable at the time of surgery
(bulky adenopathy, significant local extension/fixation). Those undergoing
PRCx were upstaged to a greater degree (63% vs. 40%) for the entire cohort
and at each level of clinical stage.

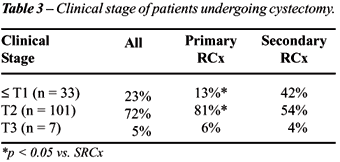
Table-3 shows the clinical stage, and Table-4
demonstrates the pathological staging of the entire cohort. Tables-3 and
4 also stratifies these results based on patients undergoing PRCx vs.
SRCx. The median time between diagnosis and cystectomy was significantly
different between the PRCx group (2 months; 0-6 months) vs. the SRCx group
(22 months; 5 - 149 months). Whereas most patients in the PRCx group were
clinical T2 or higher (87%), those patients undergoing SRCx were less
often clinical T2 or greater (58%). This difference also was observed
upon comparisons of pathological staging as well. More patients were upstaged
(63% vs. 40%), non-organ confined (62% vs. 38%), and lymph node positive
(31% vs. 20%) in the PRCx versus SRCx group.

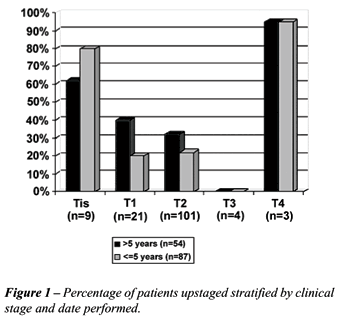
The vast majority of all patients underwent
CT imaging (11%) with the remainder of patients undergoing MRI (89%).
When patients were stratified into those staged ≤ 5 years versus
> 5 years, a greater percentage of patients underwent CT imaging before
1997 (93%) than in the most recent time period (87%). When patients were
stratified into those staged ≤ 5 years versus > 5 years there
were no significant differences between these time periods with regard
to upstaging. (Table-2 and Figure-1).
COMMENTS
It
is clear that patients with clinically-localized disease are not necessarily
a uniform population with varying outcomes with regard to operative pathology
and, accordingly, disease-free survival (DFS). For example, of patients
with clinically-localized disease, those with organ-confined disease (pT0-pT2,
N0) on surgical pathology have 5-year DFS rates exceeding 70%, while those
who are found to have non-organ-confined disease (pT3-4, N+) have rates
of less than 30%. Our inability to prospectively identify non-organ-confined
disease remains a shortcoming of current pre-operative evaluation. Consequently,
many patients (over half in the present series) are upstaged at the time
of surgical exploration and extirpation. To this end, several investigators
have sought to identify strategies to better identify and stage such patients
pre- and intra-operatively including the use of molecular and immunohistochemical
markers (e.g. p53), novel imaging techniques (e.g. photon emission computed
tomography/CT (SPECT/CT) and ferumoxtran-10-enhanced MR imaging), and
directed (e.g. sentinel node detection) or extended lymph node dissection
(5-9).
Nevertheless, several reasons may account
for this large degree of surgical upstaging, including delay between diagnosis
(i.e. TURBT) and cystectomy. Recent studies have suggested that such a
delay is associated with worse pathological findings at the time of cystectomy.
Chang et al. have shown that delaying definitive surgery more than 90
days confers a worse pathologic stage with significantly more non-organ-confined
disease (2). Similarly, Sanchez-Ortiz et al. have also demonstrated that
when cystectomy is delayed greater than 12 weeks, patients had higher
pathological stage and overall decreased survival (3). This time period
varies from patient to patient as some seek additional opinions, pursue
neoadjuvant therapies, or are completing a metastatic evaluation and clinical
staging. Nevertheless, such delays may worsen pathological and survival
outcomes, and attempts should be made to avoid such a delay.
This study sought to identify whether patients
who were delayed from undergoing their radical cystectomy due to prior
bladder-sparing therapies (SRCx) had a higher risk of understaging than
those who underwent radical cystectomy as their initial mode of treatment
(PRCx). In other words, do initial bladder sparing interventions (e.g.
intravesical therapies) contribute to increased surgical upstaging and
worse pathological outcomes as compared those who undergo immediate cystectomy?
Interestingly, this was not the case. In fact, those undergoing PRCx actually
faired worse in the both clinical and pathologic findings and were understaged
to a lesser degree, especially in clinical stage T2 and T3 disease. However,
the belief that SRCx patients fare better than PRCx patients is almost
certainly attributable to a selection bias. Patients in the SRCx group
were likely more favorable candidates from the outset: the decision to
utilize bladder-spring therapies are likely due to, in part, more favorable
clinical and pathological features. Indeed, this group had a higher percentage
of T1 (and CIS) tumors at the initial staging.
Thus, one should not extrapolate that patients
will fare better if there is a delay in definitive therapy with regard
to radical cystectomy, particularly with invasive disease. It merely suggests
that selected patients may be appropriate candidates for bladder-sparing
modalities and should not necessarily expect a worse pathologic staging
if conservative measures fail and they progress to cystectomy. Still,
despite the more favorable outcomes with regard to pathological staging
seen in the SRCx group, 40% of these patients were ultimately understaged
and 38% had pT3/T4 or node-positive disease at the time of cystectomy.
That is, those who receive delay in treatment may still be subject to
relatively high incidence of upstaging and extravesical disease on operative
pathology. Such findings suggest that some patients in this group would
fare better with earlier cystectomy.
In addition to the potential role of delay
in upstaging of patients undergoing cystectomy, limitations in the clinical
staging of invasive bladder cancer may also lead to surgical upstaging
by failing to recognize non-organ confined disease at the time of diagnosis.
The lack of an appropriate and reliable serum, urine, or other molecular
marker for bladder cancer forces reliance on standard methodology for
clinical staging. Thorough endoscopic resection of all visible tumors
(with an appropriate sampling of muscle), bimanual exam, and cross-sectional
imaging provide clues in determining the clinical course of bladder cancer.
For large volume disease, current CT and MRI can be reliable in determination
of clinical stage. Subtle invasive disease or tumors with low volume or
small nodes are less accurately staged with these imaging modalities,
however. Accordingly, multiple studies have shown disappointing correlation
between clinical and pathologic stages using existing axial imaging. Specifically,
computed tomography (CT) has been shown to have limited accuracy in correlating
clinical to pathologic stages. Paik demonstrated CT accuracy to be around
55% with understaging at 39% (10). Barentsz et al. reported CT accuracy
ranged from 40 to 92% but MRI had up to a 30% accuracy improvement over
CT (11). Low volume lymph node disease is found in a third of invasive
disease, and is it difficult to discern on CT or MRI. Herr reported two-thirds
of patients in a series with node positive disease were understaged (12).
Indeed, our data demonstrates that over half of patients are upstaged
on operative pathology, and 7% of clinical T2 patients are found to be
unresectable at the time of surgery. Ultimately, CT lacks the ability
to reliably detect small volume extravesical disease or demonstrate lymph
node metastases. Some have speculated that positron emission tomography
(PET) may be useful in prospectively distinguishing localized tumors from
regional or distant disease, but recent studies have demonstrated that
current PET imaging is at best two-thirds reliable in staging node-positive
bladder cancer disease and is still an expensive modality not found at
many tertiary centers (13-15).
Even with improved resolution of more recent
CT or MRI scanners, better outcomes with regard to clinical staging does
not seem to follow. This was evident across the time span of our retrospective
review. Despite marked advances in our radiographic modalities, which
are integral to clinical staging, we saw no difference in the stage discrepancy
in patients who were staged more than 5 years ago compared to those within
the past 5 years (Figure-1).
An important limitation of our study is
the retrospective nature of this analysis. Such an analysis does not allow
for quantification of the degree of upstaging based upon suboptimal transurethral
resection or nor does it stratify patients who underwent multiple TUR
procedures. Bayrakatar and colleagues published a series that demonstrated
substantial overstaging in TUR, which led to premature radical surgery
(16). However, Dalbagni et al. showed understaging at the time of cystectomy
was negligible in T1 disease after performing a restaging TUR procedures
(17). Still, the many differing TUR techniques employed by the referring
urologists in this study contribute to added variability of clinical staging.
In addition, a retrospective analysis does
not account for the impact of selection bias on those undergoing PRCx
versus SRCx, and makes interpretation of those results inherently imperfect
(see discussion above). Nevertheless, this report reflects the patient
population observed at a tertiary care facility at which the referral
population is often varied especially with regard to decision-making for
and selection of prior therapies.
Lastly, a large part of this analysis was
based clinical and pathologic staging without regard to tumor grade. However,
only 4 patients (< 3%) were low grade or grade-1 in this series. In
other words, the cystectomy population was almost uniformly high grade,
which thereby makes the predictive ability of tumor grade, with regard
to upstaging, unattainable.
CONCLUSION
Pathologic findings after definitive radical cystectomy for urothelial carcinoma of the bladder does not often correlate with preoperative staging. Consequently, more than half of patients undergoing cystectomy will be upstaged on their operative pathology. Patients who undergo secondary RCx (for recurrent/progressive disease after initial bladder-sparing modalities) have more favorable pathology at the time of cystectomy and are understaged to a lesser degree than patients who receive a primary radical cystectomy. However, these findings may be tempered by a selection bias that is likely found in this subgroup. Still, a significant number of these patients undergoing SRCx will be understaged and found to have extravesical disease at the time of cystectomy. An improved understanding of the relative frequency of upstaging in cystectomy patients may have important implications on the decision and selection for neoadjuvant and adjuvant therapies for these high-risk populations.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Underwood W 3rd, Dunn RL, Williams C, Lee CT: Gender and geographic influence on the racial disparity in bladder cancer mortality in the US. J Am Coll Surg. 2006; 202: 284-90.
- Chang SS, Hassan JM, Cookson MS, Wells N, Smith JA Jr: Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol. 2003; 170: 1085-7.
- Sanchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB: An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003; 169: 110-5; discussion 115.
- Tsukamoto T, Kitamura H, Takahashi A, Masumori N: Treatment of invasive bladder cancer: lessons from the past and perspective for the future. Jpn J Clin Oncol. 2004; 34: 295-306.
- Deserno WM, Harisinghani MG, Taupitz M, Jager GJ, Witjes JA, Mulders PF, et al.: Urinary bladder cancer: preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology. 2004; 233: 449-56.
- Habuchi T, Marberger M, Droller MJ, Hemstreet GP 3rd, Grossman HB, Schalken JA, et al.: Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005; 66: 64-74.
- Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, et al.: P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005; 6: 678-86.
- Sherif A, Garske U, de la Torre M, Thorn M: Hybrid SPECT-CT: an additional technique for sentinel node detection of patients with invasive bladder cancer. Eur Urol. 2006; 50: 83-91.
- Liedberg F, Chebil G, Davidsson T, Gudjonsson S, Mansson W: Intraoperative sentinel node detection improves nodal staging in invasive bladder cancer. J Urol. 2006; 175: 84-8; discussion 88-9.
- Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI: Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000; 163: 1693-6.
- Barentsz JO, Witjes JA, Ruijs JH: What is new in bladder cancer imaging. Urol Clin North Am. 1997; 24: 583-602.
- Herr HW: Routine CT scan in cystectomy patients: does it change management? Urology. 1996; 47: 324-5. Erratum in: Urology 1996 May;47(5):785.
- Shvarts O, Han KR, Seltzer M, Pantuck AJ, Belldegrun AS: Positron emission tomography in urologic oncology. Cancer Control. 2002; 9: 335-42.
- Hain SF, Maisey MN: Positron emission tomography for urological tumours. BJU Int. 2003; 92: 159-64.
- Schoder H, Larson SM: Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004; 34: 274-92.
- Bayraktar Z, Gurbuz G, Tasci AI, Sevin G: Staging error in the bladder tumor: the correlation between stage of TUR and cystectomy. Int Urol Nephrol. 2001; 33: 627-9.
- Dalbagni G, Herr HW, Reuter VE: Impact of a second transurethral resection on the staging of T1 bladder cancer. Urology. 2002; 60: 822-4; discussion 824-5.
_____________________
Accepted
after revision:
September 25, 2006
______________________
Correspondence address:
Dr. Raj S. Pruthi
Division of Urologic Surgery
The University of North Carolina at Chapel Hill
2140 Bioinformatics Bldg, CB7235
Chapel Hill, North Carolina 27599, USA
E-mail: rpruthi@med.unc.edu
In
this retrospective study, authors demonstrate the difficulties in accurate
preoperative clinical staging in patients undergoing radical cystectomy
(RC). This study highlights several important issues. Firstly, there are
no reliable diagnostic tools available to accurately stage bladder cancer
(BC). There is an urgent need to develop accurate imaging studies and
markers to stage the disease accurately and predict the prognosis. Secondly,
this study demonstrates that more than 50% of patients undergoing RC are
under staged and perhaps unfairly denied neoadjuvant chemotherapy. This
may support the argument that all patients with ≥ T2 bladder cancer
may benefit from neoadjuvant chemotherapy (1).
Timing of RC in patients with CIS and T1 high grade bladder cancer is
controversial (2). Accurate staging of patients undergoing intravesical
BCG therapy and in those who failed BCG therapy is important as studies
have demonstrated disease progression to higher stage (> T2) in patients
undergoing RC (3,4).
Until accurate imaging studies and markers
are available, thorough bimanual examination during transurethral resection
remains an important staging tool. As authors suggested patients should
be formally counseled regarding potential under staging preoperatively.
REFERENCES
- Sawhney R, Bourgeois D, Chaudhary UB: Neo-adjuvant chemotherapy for muscle-invasive bladder cancer: a look ahead. Ann Oncol. 2006; 17: 1360-9.
- Manoharan M, Soloway MS: Optimal management of the T1G3 bladder cancer. Urol Clin North Am. 2005; 32: 133-45.
- Nieder AM, Simon MA, Kim SS, Manoharan M, Soloway MS: Radical cystectomy after bacillus Calmette-Guerin for high-risk Ta, T1, and carcinoma in situ: defining the risk of initial bladder preservation. Urology. 2006; 67: 737-41.
- Higashi S, Matsui Y, Takahashi T, Nishiyama H, Ito N, Yamamoto S, Kamoto T, Ogawa O: [The influence over the long-term prognosis of BCG therapy and the surgical treatment in superficial bladder cancer treatment]. Hinyokika Kiyo. 2005; 51: 529-31.
Dr. M. Manoharan
Director, Bladder Cancer & Neobladder Center
Miller School of Medicine
University of Miami
Miami, Florida, USA
E-mail: mmanoharan@med.miami.edu