WET
HEAT EXPOSURE: A POTENTIALLY REVERSIBLE CAUSE OF LOW SEMEN QUALITY IN
INFERTILE MEN
(
Download pdf )
SHAI SHEFI, PHIROZ E. TARAPORE, THOMAS J. WALSH, MARY CROUGHAN, PAUL J. TUREK
Departments of Urology (SS, PET, TJW, PJT), Obstetrics, Gynecology and Reproductive Science (MC, PJT), and Epidemiology and Biostatistics (MC), University of California San Francisco, San Francisco, California, USA
ABSTRACT
Objective:
To evaluate the recovery of semen quality in a cohort of infertile men
after known hyperthermic exposure to hot tubs, hot baths or whirlpool
baths.
Materials and Methods: A consecutive cohort
of infertile men had a history remarkable for wet heat exposure in the
forms of hot tubs, Jacuzzi or hot baths. Clinical characteristics and
exposure parameters were assessed before exposure was discontinued, and
semen parameters analyzed before and after discontinuation of hyperthermic
exposure. A significant seminal response to withdrawal of hyperthermia
was defined as ≥ 200% increase in the total motile sperm count (TMC
= volume x concentration x motile fraction) during follow-up after cessation
of wet heat exposure.
Results: Eleven infertile men (mean age
36.5 years, range 31-44) exposed to hyperthermia were evaluated pre and
post-exposure. Five patients (45%) responded favorably to cessation of
heat exposure and had a mean increase in total motile sperm counts of
491%. This increase was largely the result of a statistically significant
increase in sperm motility from a mean of 12% at baseline to 34% post-intervention
(p = 0.02). Among non-responders, a smoking history revealed a mean of
5.6 pack-years, compared to 0.11 pack-years among responders. The prevalence
of varicoceles was similar in both cohorts.
Conclusions: The toxic effect of hyperthermia
on semen quality may be reversible in some infertile men. We observed
that the seminal response to exposure elimination varies biologically
among individuals and can be profound in magnitude. Among non-responders,
other risk factors that could explain a lack of response to elimination
of hyperthermia should be considered.
Key
words: male infertility; induced hyperthermia; semen; analysis;
spermatogenesis
Int Braz J Urol. 2007; 33: 50-7
INTRODUCTION
Spermatogenesis
is sensitive to a variety of chemical and physical stressors. Testicular
hyperthermia has been known to have a deleterious effect on male fertility
since the time of Hippocrates and is a well-recognized cause of impaired
sperm production (1). Its detrimental effect has been demonstrated in
both animal models and in humans (2-4). Whether due to endogenous (such
as high fevers) or exogenous stimuli, heat decreases sperm concentration,
impairs motility, and reduces the number of morphologically normal sperm
(5-8). This effect is striking enough that the effect of laptop computers
on scrotal hyperthermia has recently been reported and the use of heat
exposure as a male contraceptive has been studied (9,10). In contrast
to the well described detrimental effects of dry heat on spermatogenesis,
the consequences of wet heat exposure are relatively undefined. Our working
hypothesis is that the effects of wet heat on spermatogenesis are similar
to that from dry heat.
The only published study to examine the
effects of wet heat exposure on human fertility was performed by Rock
and Robinson in 1965. In this study, the authors exposed 20 oligospermic
men to wet heat (43-45°C) with a bottle warmer held between the thighs
for 30 minutes on 6 alternating days. They noted a decline in sperm production
that was followed by improvement in seminal parameters with cessation
of the exposure, however the details of semen quality before and after
exposure were not provided by the authors (11). The effects of more common
exposures to wet heat, such as with hot tubs, Jacuzzis, or hot baths have
not been addressed in the literature.
The data provided by Rock and Robinson,
as well as the well-defined association between dry heat exposure and
impaired spermatogenesis led us to study the effect of total-body wet
heat exposure in human males. We asked whether withdrawal of exposure
in men with poor semen quality and a history of wet heat exposure could
lead to an improvement in semen quality. We retrospectively compared semen
quality before and after cessation of wet heat exposure, and also performed
an interval analysis of responders and non-responders, looking for factors
that might modulate responsiveness to therapeutic intervention.
MATERIALS AND METHODS
Over
a 3-year period, infertile men with exposure to wet heat were identified
in a single, university-based, male infertility practice. Inclusion criteria
were wet heat exposure, defined as the immersion of the body in a hot
tub, heated Jacuzzi, or bath at a temperature warmer than body temperature,
for ≥ 30 minutes per week during ≥ 3 months prior to presentation.
All female partners were concurrently assessed for reproductive issues.
Patients were excluded if they had received any medical or surgical infertility
treatment within 1 year of study intake, and if a diagnosis of co-existing
female factor infertility was made. Because patient data was decoded of
all protected health information, a waiver was obtained from the Committee
on Human Research for this study.
All subjects underwent a complete history
and physical examination by a single specialist (PJT). Information regarding
the type, frequency, duration/episode and overall length of exposure to
wet heat was collected during this visit. Counseling regarding the cessation
of wet heat exposure was given during the initial visit. We attempted
to obtain 2 semen samples for analysis at baseline (during exposure),
< 3 months after intervention, and between 3-6 months after intervention
in all patients. Semen was collected by masturbation after 2 to 3 days
of abstinence and processed within 1 hour of ejaculation. All semen analyses
were performed in a single andrology laboratory by the same technician,
and assessed according to World Health Organization guidelines (12). Motility
was reported as the sum of A + B patterns (12). A positive response to
discontinuation of heat exposure was defined as an increase in total motile
count (TMC = ejaculate volume x sperm concentration x fraction of motile
sperm) > 200% during follow-up. This cutoff was selected to be above
the natural variability in semen parameters within an individual: sperm
volume, concentration and motility have been shown to vary by 59%, 54%
and 96%, respectively, in a single individual with time (13). Statistical
analysis was performed using two-tailed, paired “t” test to
compare pre- and post-intervention seminal parameters. Probability values
< 0.05 were considered significant.
RESULTS
Patient
Characteristics
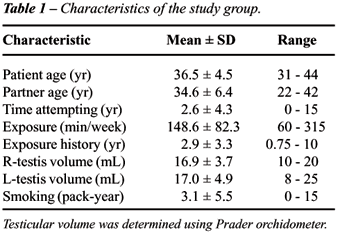
Table-1 outlines the demographic and clinical
characteristics of the 11 eligible study subjects. Mean patient age was
36.5 years, and mean partner age was 34.6 years. Couples had been trying
to conceive for an average of 2.6 years (range 0 - 15 years) prior to
evaluation. Nine patients (82%) had an evidence of infertility, defined
as failure to conceive after one year of unprotected sexual intercourse.
The mean exposure duration was 149 minutes per week (range 60 - 315),
with a mean total duration of hyperthermic exposure of 2.9 years (range
0.75 - 10). Five subjects were exposed to hot baths, 4 to both hot baths
and hot tubs, and 2 to Jacuzzi. Three of the 11 subjects had a significant
smoking history (> 6 pack-years). Five others were only occasional
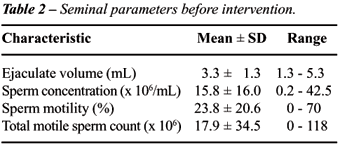
smokers (< 1 pack-years). Complete pre-intervention semen parameters
among study subjects are given in Table-2. Patients had an average of
1.6 ± 0.6 and 2.5 ± 1.7 semen analyses performed prior to
cessation of exposure and following cessation, respectively. Nine men
(82%) had TMC < 20 x 106 (mean 5 x 106). The other 2 men
had TMC’s of 34 x 106 and 117.6 x 106 sperm.
Response
to Cessation of Wet Heat Exposure
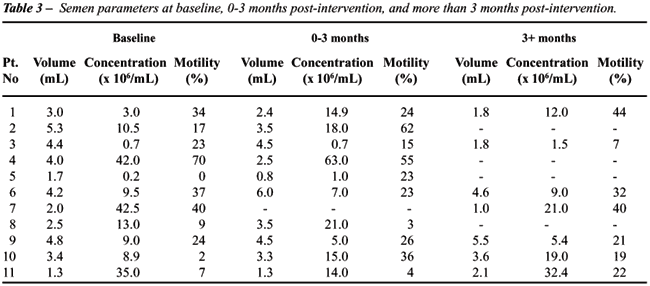
Semen analyses for each subject were categorized
into baseline (during exposure), 0 to 3 months post-intervention, and
> 3 months post-intervention. Multiple semen analyses within a given
period were averaged for each individual during that period. Sperm parameters
before and after discontinuation of exposure are listed in Table-3. TMC
values and response to intervention status are shown in Table-4. Mean
follow-up of the study cohort was 7 months (range 2-16). Amongst the entire
cohort, improvement in TMC did not reach statistical significance (p =
0.89). Overall, 44.5 % (95% CI 16.7,76.7) of men showed > 200% increase
in total motile sperm count after discontinuation of wet heat exposure
and were considered responders to intervention.
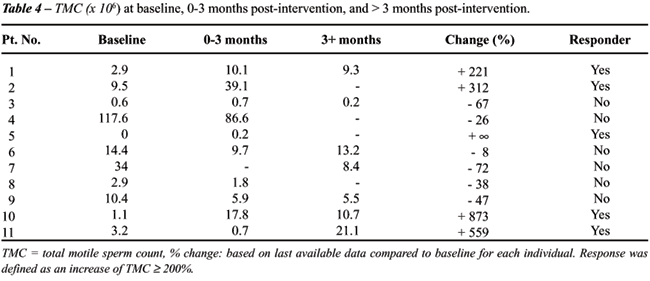
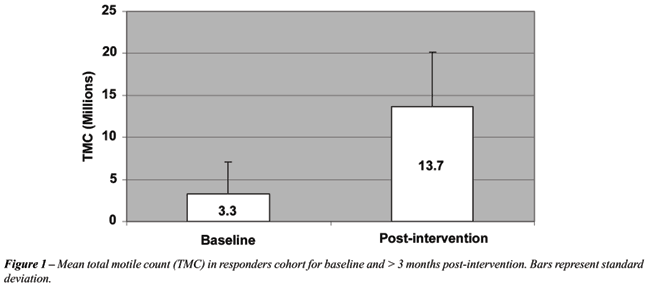
Among responders, the mean increase in TMC
was 491% (range 221-873), excluding an outlier whose TMC improved from
zero to 200,000 sperm. Responders’ TMC before and after intervention
are illustrated in Figure-1. The seminal response in the responding cohort
showed increases in sperm concentration, but largely the result of increases
in sperm motility. Although the increase in TMC increase did not reach
statistical significance (p = 0.07) among responders, a subset analysis
showed a mean increase of 22% in sperm motility, which was statistically
significant (p = 0.02). Among responders, one patient had a TMC increase
from 0 to 0.2 million sperm, two patients had post-intervention TMC’s
of 9.3 and 10.7 x 106, while two more had a TMC’s of
> 20 x 106 sperm. There was no significant difference in
the length of follow-up among study responders and non-responders.
Factors that could explain a response or
lack of response to the intervention were examined in both cohorts. Tobacco
use emerged as a possible differentiating factor. Among 6 non-responders,
5 were chronic tobacco users with a significant smoking history (mean
5.6 pack-years), compared with 3 occasional smokers in the responder group,
whose mean tobacco consumption was 0.11 pack-years. The prevalence of
varicoceles was similar in responders and non-responders (4/5 and 4/6
patients, respectively). No other potential gonadotoxic factors were identified
in the study cohort.
COMMENTS
The
removal of wet heat exposure resulted in improvement in semen quality
in nearly one-half of subjects studied. The improvement was largely the
results of increases in sperm motility, and appeared to persist beyond
3 months, although a continuous improvement was not observed in all subjects.
The extra-abdominal position of the scrotum and the proximity of the pampiniform
venous plexus to the testicular arteries contribute to the efficient dissipation
and transfer of heat away from the testes and resultant lower testicular
temperature (8). However, in the presence of extreme elevation of extra-testicular
temperature, as when immersed in hot liquid, these same characteristics
make the scrotum particularly susceptible to deleterious thermal effect.
It is therefore not surprising that semen quality might be susceptible
to the effects of wet hyperthermia.
The deleterious effect of dry heat on semen
quality and, by extrapolation, male fertility, has been recognized medically
for decades, and to the traditional medicine community for millennia.
Carlsen et al., in examining the effect of febrile illness on semen quality,
demonstrated a dose-response relationship between the number of days with
fever and sperm concentration (8). Similarly, Mieusset & Bujan examined
mild testicular heating (about 1°C) as a form of contraception, an
idea originally conceived of by Robinson et al. (14,15). Both studies
found that sperm concentration rebounded to baseline in 12 to 18 months
following cessation of increased testicular temperatures. None of these
studies, however, were designed to replicate the frequent, significant
wet hyperthermia that is the fate of the habitual hot-tuber, thus forming
the rationale for this study.
In reviewing our data on the recovery of
semen quality in infertile males following the cessation of wet heat exposure,
we found that there were two distinct groups of patients: those that responded
to intervention and those that did not. Among responders, improvements
in semen quality were witnessed well beyond the 3 month period typically
ascribed to a single cycle of spermatogenesis. This finding is entirely
consistent with the time course of recovery noted after varicocele repair
and exposure to other gonadotoxins (16). Interestingly, the semen analysis
parameter that exhibited the largest change among responders was sperm
motility, and this increase reached statistical significance after intervention.
This suggests that heat-induced motility dysfunction may be the parameter
most vulnerable to wet hyperthermic exposure. Although statistical significance
was not achieved in the overall increase in TMC after intervention, the
changes observed could be considered clinically significant, as more men
could qualify for IUI instead of IVF for infertility treatment following
intervention. Given that intrauterine insemination (IUI) can be considered
for low semen quality in men with TMC > 8-10 x 106 sperm,
the number of men who would qualify for IUI in the responder group increased
from 1/5 pre-intervention to 4/5 post-intervention. Two of the responders
had TMC > 20 x 106 after intervention and were advised to
consider unprotected intercourse before IUI. This “shift of care”
to less intensive forms of assisted reproduction has also been described
for varicocele repair (16). We acknowledge that even statistically significant
changes in semen parameters are always problematic to assess, given the
naturally high intra-individual variability of semen parameters in both
fertile and infertile men. However, the potential clinical value of these
changes must also be considered, as we have outlined.
Careful analysis of non-responders suggests
that chronic tobacco use was more common among non-responders, suggesting
that it may complicate the recovery from wet heat exposure. This finding
is hardly surprising, considering that tobacco use is a well established,
independent risk factor for poor semen quality (17-19). It is interesting
to speculate whether the concomitant exposure to tobacco and wet heat
may act synergistically to prevent early and rapid recovery of semen quality.
It is interesting that two responders demonstrated
subtle declines in TMC following the 3-month time interval. This lack
of continuous improvement suggests that peak improvements in TMC may be
time-limited and could involve other, less well-described physiological
responses. It may also suggest that a timeline for therapy be offered
to affected patients, to avoid prolonged follow-up without obvious benefit.
Because this is a small study, the results
should be confirmed in a larger series of patients. Given the complexity
of factors underlying male infertility, including currently uncharacterized
factors, more accurate results will only be generalizable with a larger
study. We plan further follow-up on the patients in this study to assess
conception after elimination of hyperthermia.
CONCLUSIONS
This study addressed the relatively unsubstantiated issue of wet heat exposure as a factor in male infertility. We demonstrated that infertile men who are frequently exposed to wet heat in the form of hot tubs, Jacuzzis, or hot baths, may realize a marked increase in semen quality following cessation of exposure. The response persists for more than 3 months, and is driven mostly by the increase in sperm motility.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENT
Doctor Shai Shefi is Recipient of fellowship from The American Physicians Fellowship for Medicine in Israel.
REFERENCES
- Dada R, Gupta NP, Kucheria K: Spermatogenic arrest in men with testicular hyperthermia. Teratog Carcinog Mutagen. 2003; Suppl 1: 235-43.
- Lue YH, Lasley BL, Laughlin LS, Swerdloff RS, Hikim AP, Leung A, et al.: Mild testicular hyperthermia induces profound transitional spermatogenic suppression through increased germ cell apoptosis in adult cynomolgus monkeys (Macaca fascicularis). J Androl. 2002; 23: 799-805.
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ: Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001; 65: 229-39.
- Adams F (translator):The Genuine Works of Hippocrates. Baltimore, Wilkins & Wilkins. 1939.
- MacLeod J, Hotchkiss RS: The effect of hyperpyrexia upon. spermatozoa counts in men. Endocrinology. 1941; 28: 780–784.
- Procope BJ: Effect of repeated increase of body temperature on human sperm cells. Int J Fertil. 1965; 10: 333-9.
- Saikhun J, Kitiyanant Y, Vanadurongwan V, Pavasuthipaisit K: Effects of sauna on sperm movement characteristics of normal men measured by computer-assisted sperm analysis. Int J Androl. 1998; 21: 358-63.
- Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE: History of febrile illness and variation in semen quality. Hum Reprod. 2003; 18: 2089-92.
- Sheynkin Y, Jung M, Yoo P, Schulsinger D, Komaroff E: Increase in scrotal temperature in laptop computer users. Hum Reprod. 2005; 20: 452-5.
- Kandeel FR, Swerdloff RS: Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril. 1988; 49: 1-23.
- Rock J, Robinson D: Effect of induced intrascrotal hyperthermia on testicular function in man. Am J Obstet Gynecol. 1965; 93: 793-801.
- WHO Laboratory Manual for the Examination of the Human Semen and Sperm-Cervical Mucus Interaction. Fourth edition. Cambridge, Cambridge University Press. 1999.
- Mallidis C, Howard EJ, Baker HW: Variation of semen quality in normal men. Int J Androl. 1991; 14: 99-107.
- Mieusset R, Bujan L: The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int J Androl. 1994; 17: 186-91.
- Robinson D, Rock J, Menkin MF: Control of human spermatogenesis by induced changes of intrascrotal temperature. JAMA. 1968; 204: 290-7.
- Cayan S, Erdemir F, Ozbey I, Turek PJ, Kadioglu A, Tellaloglu S: Can varicocelectomy significantly change the way couples use assisted reproductive technologies? J Urol. 2002; 167: 1749-52.
- Hull MG, North K, Taylor H, Farrow A, Ford WC: Delayed conception and active and passive smoking. The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000; 74: 725-33.
- Vine MF: Smoking and male reproduction: a review. Int J Androl. 1996; 19: 323-37.
- Hughes EG, Brennan BG: Does cigarette smoking impair natural or assisted fecundity? Fertil Steril. 1996; 66: 679-89.
____________________
Accepted after revision:
October 18, 2006
_______________________
Correspondence address:
Dr. Paul J Turek
Department of Urology, UCSF
1600 Divisidero St, Rm A633
San Francisco, CA, 94143-1695, USA
E-mail: pturek@urology.ucsf.edu