OUTCOMES
FOLLOWING NEGATIVE PROSTATE BIOPSY FOR PATIENTS WITH PERSISTENT DISEASE
AFTER RADIOTHERAPY FOR PROSTATE CANCER
(
Download pdf )
doi: 10.1590/S1677-55382010000100007
JACOB H. COHEN, JAMES EASTHAM, RICHARD J. MACCHIA
Department of Urology (JHC, RJM), State University of New York Downstate Medical School, Brooklyn, New York, USA, and Memorial Sloan-Kettering Cancer Center (JE), New York, New York, USA
ABSTRACT
Purpose:
When faced with biochemical recurrence after definitive radiotherapy for
prostate cancer, clinicians must determine whether the recurrence is local
or systemic. Post radiotherapy prostate biopsies to detect persistent
local disease are difficult to interpret histopathologically and are subject
to sampling error. Our study examines outcomes for patients with a negative
prostate biopsy performed for rising prostate-specific antigen (PSA) levels
after prostate radiation.
Materials and Methods: We performed a retrospective
review of 238 prostate cancer patients with a negative biopsy following
definitive radiotherapy. Seventy-five of these patients had biochemical
recurrence at the time of biopsy. A negative biopsy was defined as the
absence of prostate cancer without radiation-treatment effect in the specimen.
Results: Patients underwent biopsy at a
mean of 41 months after the completion of radiation. They had a mean PSA
of 6. Patients were followed for an average of 63 months. Thirty-two patients
(43%) developed metastasis, and 11 (15%) died of prostate cancer despite
a negative post-radiation biopsy. Five of nine patients (56%) with sequential
biopsies had a positive second biopsy.
Conclusions: Patients with PSA recurrence
and a negative post-radiation biopsy have a high chance of persistent
local disease, progression, and death from prostate cancer. Furthermore,
an initial negative biopsy does not rule-out local recurrence. Patients
with biochemical recurrence after radiotherapy for prostate cancer need
to be evaluated earlier for local recurrence.
Key
words: prostate neoplasms; prostate-specific antigen; neoplasm
recurrence; radiation
Int Braz J Urol. 2010; 36: 44-8
INTRODUCTION
Biochemical recurrence after radiotherapy for prostate cancer occurs in approximately 10-60% of patients and varies depending on definition of recurrence, tumor stage and grade at the time of diagnosis, dosage of radiation, and the use of adjuvant hormonal therapy (1). Salvage therapy for persistent local disease after radiotherapy has shown greatest efficacy for biopsy-proven local recurrence with low prostate-specific antigen (PSA) level, and negative metastatic evaluation (2). Post-radiotherapy prostate biopsy to detect persistent local disease is difficult to interpret histopathologically and subject to sampling error. In addition, there are no well-defined recommendations for when or how to biopsy these patients. Most studies have examined post-radiotherapy biopsies regardless of signs or symptoms or disease progression. Our study is the first, to our knowledge, to examine outcomes for patients with a negative prostate biopsy performed for biochemical recurrence after radiation.
MATERIALS AND METHODS
We performed a retrospective review of 238 prostate cancer patients in a prospectively maintained prostate cancer database who had a negative prostate biopsy following definitive radiotherapy between January 1st, 1992 and December 31st, 2005. Of these patients, 155 underwent prostate biopsy as part of a clinical trial without evidence of biochemical failure, while 83 patients were identified to have biochemical recurrence at the time of their post-radiotherapy biopsy. Eight patients were excluded due to missing data or lack of follow-up, leaving 75 patients for analysis. Biochemical recurrence was determined by the treating practitioner, most typically the American Society for Therapeutic Radiology and Oncology (ASTRO) definition of biochemical failure (3). A negative post-radiation biopsy included a pathology report of benign tissue, benign tissue with profound treatment effect, or prostate cancer with profound treatment effect (4).
RESULTS
Our 75 patients had a mean age of 66 years and a mean PSA of 15 ng/mL at initial cancer diagnosis. Table-1 shows Gleason score and clinical stage at initial cancer diagnosis as well as initial treatment. A post-radiotherapy PSA-nadir of < 1.0 was achieved in 69 patients (92%). Patients underwent biopsy at a mean of 41 months after the completion of radiation and with a mean PSA of 6 ng/mL. Mean and median PSA doubling time at post-radiotherapy biopsy were 12 and 9 months, respectively. For the 55 (73%) patients on whom data were available, there was no standard technique for post-radiotherapy biopsy, with as little as 2 cores and as many as 24 cores sampled. Mean follow-up after the negative post-radiotherapy biopsy was 63 months.
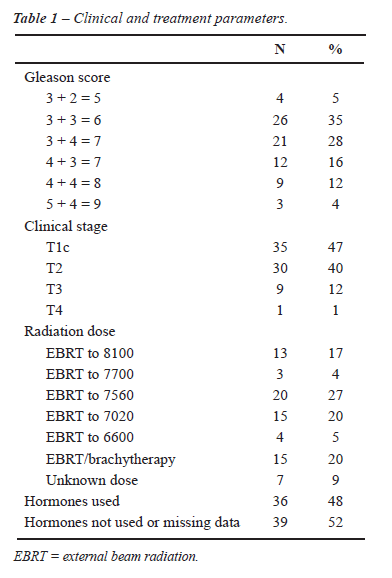
Patient outcomes following their negative
post-radiation biopsy are presented in Figure-1. There were nine patients
undergoing two sequential post-radiotherapy biopsies. Five of nine (56%)
had their second biopsy return positive, and all five were alive after
their salvage local therapies. Of the four patients who had two sequential
negative post-radiotherapy biopsies, there was one death from prostate
cancer, one patient with clinical metastasis, one patient with asymptomatic
metastasis, and one patient with biochemical recurrence alone.
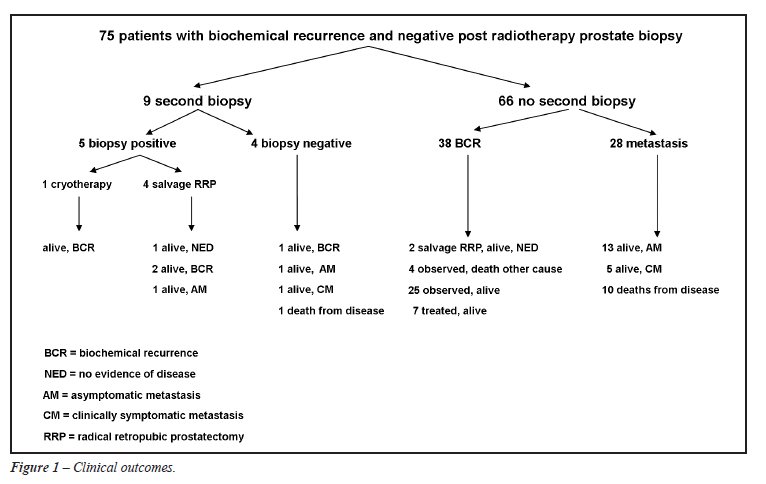
Overall, 32 patients (43%) developed disease
progression beyond biochemical recurrence, with 15 (20%) developing radiographic
metastasis only, 6 (8%) developing clinically symptomatic metastasis,
and 11 (15%) dying from prostate cancer. These patients had a mean PSA
of 9 at the time of re-biopsy, with a mean PSA-doubling time of 9 months.
Twenty-nine patients (39%) had biochemical
recurrence only and were observed. Their mean PSA at the time of re-biopsy
was 4, with a mean PSA-doubling time of 15 months. This group of patients
was followed for a mean 56 months after re-biopsy, and the mean PSA at
last follow-up was 11 with four deaths from other causes.
COMMENTS
Post-radiotherapy
biopsies are very complex to interpret and depend on the experience of
the reading pathologist, the elapsed time interval following radiotherapy,
the presence or absence of concomitant androgen therapy, the total dose
of radiotherapy administered, and the amount and degree of treatment effect.
Radiotherapy is known to induce a variety of histological changes in normal
and cancerous prostate tissue, including atrophy, cytology atypia, mucinous
metaplasia, cytoplasmic vacuolization, and diminution of neoplastic glands
(4).
Complicating matters, the effect of radiation on prostate cancer cells
changes over time. Crook et al. prospectively studied 498 patients who
underwent systematic 6-core trans-rectal ultrasound-guided prostate biopsies
at standard intervals following radiotherapy (at 12 months post radiation
and every 6-12 months thereafter) (5). Thirty percent of patients with
an initially positive first post-treatment biopsy at 12 months eventually
converted to a negative biopsy at a mean time of 30 months. Indeterminate
biopsies (those with profound treatment effect) at first post-treatment
biopsy converted to negative in 30% and progressed to local failure in
18%. Finally, 19% of those with an initially negative post-treatment biopsy
were found to have residual local disease at systematic 36-month biopsy.
The authors concluded that the greatest predictive value of a post-radiation
biopsy is between 30 and 36 months, and that biopsies with profound treatment
effect (intermediate category) should be repeated as residual radiated
tumor eventually declares its biological activity over time.
Positive re-biopsy rates have also been shown to correlate with radiation
dose. Liebel et al. found lower positive re-biopsy rates at 2.5 years
after radiation for higher radiation doses, with 57% positive re-biopsy
rate at 64.8 Gy, 44% at 70.2 Gy, 45% at 75.6 Gy, and 7% at 81 Gy (6).
Zelefsky et al. also corroborated these findings (7). In contrast, Pollack
et al. found equal positive re-biopsy rates when comparing 70-Gy to 78-Gy
(8).
In 1999, ASTRO published a consensus statement regarding guidelines for
re-biopsy after radiation (9). This group concluded that systematic prostate
re-biopsy is not a standard of care for prostate cancer patients, that
it should only be considered for patients who are candidates for effective
salvage local therapy, and that it should be performed at least two years
following the conclusion of radiation therapy. PSA guidelines were not
given in this statement. A later consensus statement showed the newer
Phoenix definition of recurrence (nadir plus 2) to predict for metastatic
failure, implying that biopsies to detect persistent local disease should
occur before “nadir plus 2” is reached (10).
Many studies have shown increased incidence of local recurrence, distant
metastasis, and death from prostate cancer for those patients with a positive
post-radiation biopsy (11,12). Our retrospective study specifically examined
outcomes for patients with a negative post-radiation prostate biopsy.
Decision to re-biopsy was at the discretion of the treating physician,
but all patients had a rising PSA at the time of re-biopsy.
The patients in our study had generally poor outcomes, with 32 (43%) developing
clinical disease progression. All of these patients were treated with
hormonal therapy, and an additional 14 patients received hormonal therapy
based on biochemical recurrence alone. Indeed, our patients had high PSA
levels at the time of re-biopsy (mean 6) and high PSA doubling times at
the time of re-biopsy (mean 12 months), indicating that the initiation
of the search for persistent local disease occurred relatively late in
the disease process. Not surprisingly, those patients with the worst outcomes
(radiographic or clinical metastasis or death from prostate cancer), had
higher mean PSA (9) and shorter mean PSA-doubling time (9 months) at the
time of their biopsy. Another possible contributing factor to the diverse
clinical outcomes we observed might be the wide range of radiotherapy
doses used during initial treatment, as well as whether neoadjuvant or
adjuvant hormonal therapy was used.
Our retrospective review does not allow definitive statements regarding
the prognostic value of a negative post-radiation biopsy performed for
biochemical recurrence. However, it is interesting that despite five of
nine patients (56%) of those undergoing multiple biopsies being upgraded
to cancer on repeat biopsy, all of these patients were successfully salvaged
with local therapy, and two of these patients had nodal metastasis at
the time of salvage prostatectomy. Had these patients not been re-biopsied,
they may not have been offered successful local/regional salvage therapy,
and their disease outcome would have likely suffered.
In conclusion, we documented high rates of disease progression and eventual
death from prostate cancer in a group of 75 men who had rising PSA after
radiotherapy but a negative post-radiation prostate biopsy. In future
studies, we will compare outcomes to patients in the same database with
positive post-radiation prostate biopsies. In clinical practice, we believe
men with rising PSA after radiation should be offered a systematic prostate
biopsy to document persistent local disease and offer the possibility
of cure with additional local/regional therapy.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA: Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1-T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004; 100: 1283-92.
- Link P, Freiha FS: Radical prostatectomy after definitive radiation therapy for prostate cancer. Urology. 1991; 37: 189-92.
- No authors listed: Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997; 37: 1035-41.
- Gaudin PB, Zelefsky MJ, Leibel SA, Fuks Z, Reuter VE: Histopathologic effects of three-dimensional conformal external beam radiation therapy on benign and malignant prostate tissues. Am J Surg Pathol. 1999; 23: 1021-31.
- Crook J, Malone S, Perry G, Bahadur Y, Robertson S, Abdolell M: Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys. 2000; 48: 355-67.
- Leibel SA, Zelefsky MJ, Kutcher GJ, Burman CM, Mohan R, Mageras GS, et al.: The biological basis and clinical application of three-dimensional conformal external beam radiation therapy in carcinoma of the prostate. Semin Oncol. 1994; 21: 580-97.
- Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, et al.: High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001; 166: 876-81. Erratum in: J Urol. 2001; 166: 1839.
- Pollack A, Zagars GK, Antolak JA, Kuban DA, Rosen II: Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002; 54: 677-85.
- Cox JD, Gallagher MJ, Hammond EH, Kaplan RS, Schellhammer PF: Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol. 1999; 17: 1155.
- Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al.: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006; 65: 965-74.
- Kuban DA, el-Mahdi AM, Schellhammer P: The significance of post-irradiation prostate biopsy with long-term follow-up. Int J Radiat Oncol Biol Phys. 1992; 24: 409-14.
- Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A: Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008; 179: 1368-73; discussion 1373.
____________________
Accepted after revision:
October 5, 2009
_______________________
Correspondence address:
Dr. Jacob H. Cohen
Division of Urology
State University of New York
Downstate Medical School
Brooklyn, NY, 11203, USA
Fax: + 1 718 270-3848
E-mail: jhcohen4@gmail.com