LEARNING
CURVE FOR RADICAL RETROPUBIC PROSTATECTOMY
(
Download pdf )
Clinical Urology
Vol. 37 (1): 67-78,
January - February, 2011
doi: 10.1590/S1677-55382011000100009
FERNANDO J. A. SAITO, MARCOS F. DALL’OGLIO, GUSTAVO X. EBAID, HOMERO BRUSCHINI, DAHER C. CHADE, MIGUEL SROUGI
Division of Urology, University of Sao Paulo Medical School and Cancer Institute, State of Sao Paulo, Sao Paulo, Brazil
ABSTRACT
Purpose:
The learning curve is a period in which the surgical procedure is performed
with difficulty and slowness, leading to a higher risk of complications
and reduced effectiveness due the surgeon’s inexperience. We sought
to analyze the residents’ learning curve for open radical prostatectomy
(RP) in a training program.
Materials and Methods: We conducted a prospective
study from June 2006 to January 2008 in the academic environment of the
University of São Paulo. Five residents operated on 184 patients
during a four-month rotation in the urologic oncology division, mentored
by the same physician assistants. We performed sequential analyses according
to the number of surgeries, as follows: = 10, 11 to 19, 20 to 28, and
= 29.
Results: The residents performed an average
of 37 RP each. The average psa was 9.3 ng/mL and clinical stage T1c in
71% of the patients. The pathological stage was pT2 (73%), pT3 (23%),
pT4 (4%), and 46% of the patients had a Gleason score 7 or higher. In
all surgeries, the average operative time and estimated blood loss was
140 minutes and 488 mL. Overall, 7.2% of patients required blood transfusion,
and 23% had positive surgical margins.
Conclusion: During the initial RP learning
curve, we found a significant reduction in the operative time; blood transfusion
during the procedures and positive surgical margin rate were stable in
our series.
Key
words: prostatic neoplasms; prostatectomy; learning; internship
and residency; postoperative complications
Int Braz J Urol. 2011; 37: 67-78
INTRODUCTION
Prostate
cancer (PCa) is currently the most common malignant tumor among men in
Europe and the United States (US), except for malignant non-melanoma skin
tumors. In the US, it is estimated that about 192,280 new cases are diagnosed
per year, with 27,360 deaths a year due to PCa, which represents 9% of
all cancer deaths in the country per year (1). In Europe, each year there
are an estimated 190,000 new cases, with more than 50,000 deaths from
the disease (2).
Radical prostatectomy (RP) was the first
widely used standard treatment for localized PCa. The classic approach
is the retropubic technique. RP was introduced in 1905 by Young and reviewed
by Millin in 1946. However, it only became routinely and safely performed
in 1982, when Walsh et al. published new technical aspects of the surgery,
definitely setting the surgical standards for the treatment of PCa (3).
Since then, new techniques and approaches have been developed, such as
perineal (4), laparoscopic (5,6) and robotic-assisted RP (7). Throughout
the first decade of the 21st century, the use of robotic-assisted surgery
has rapidly increased in the U.S. (1), spanning the last three years to
Europe (2) and finally to Brazil in 2008 (8).
Subsequently new technological elements
have been incorporated into the surgical technique of RP, and increasingly
high additional direct and indirect expenses have significantly added
to the total cost of the procedure. Notwithstanding
the problem of significantly elevated costs, technological complexity
incorporated into new techniques may result in a longer or yet unclear
learning curve (9).
High costs and a possibly longer learning
curve prompted us to question the applicability of these new surgical
modalities into clinical practice of our hospitals, especially those related
to the public health system of our country. Furthermore, there still lacks
a thorough discussion of their unclear benefits to oncologic outcomes
and quality of life of patients who undergo minimally invasive procedures
(10). To what extent have perineal, laparoscopic or robotic-assisted RP
proved superior to the open retropubic approach?
The learning curve in surgery can be defined
as the number of cases required to perform the procedure with reasonable
operating time and an acceptable rate of complications, resulting in an
adequate postoperative clinical outcome associated with a shorter hospital
stay. Obviously, several key factors may impact the learning curve, not
only such as those related to the surgeon, as attitude, confidence, experience
with other surgical procedures, but also those related to the team members
involved in the procedures. Undoubtedly, the number of cases performed
by the surgeon and the volume of surgeries in a given center may certainly
delineate the course of surgical outcomes (11).
RP is a particularly complex surgical procedure
and it is assumed to be closely related to the surgical technique employed,
depending in part on the surgeon’s experience. Currently each RP
technique, either open (retropubic and perineal), or minimally invasive
(laparoscopic and robotic), present distinctive learning curves for the
surgeon.
Due to the wide variation in training formats
offered in the various surgical programs in urology, we sought to evaluate
the learning curve for open RP among third-year urology residents (fifth
year of residency in surgery overall) in a high volume tertiary referral
center. We aimed at both defining a minimum number of procedures necessary
to properly train the resident surgeon in urology for this procedure,
as well as on determining the most sensitive key points of the learning
process. As a result, we may be able to continuously improve the teaching
process of the surgical technique and make it widely available to mentors
and teaching centers, especially considering the social environment of
growing ethical concerns with patient safety.
MATERIALS AND METHODS
We
conducted a prospective study from June 2006 to January 2008 in the urologic
oncology division of the University of São Paulo. Patients with
clinically localized prostate adenocarcinoma (cT1-2 Nx M0) with medical
conditions for surgical treatment were selected. Five residents operated
on 184 patients during a four-month rotation in the urologic oncology
division, mentored by the same physician assistants. Patients who had
undergone other treatments such as chemotherapy, radiation therapy or
biological agents prior or concomitant to surgery and patients with significant
neurological, psychiatric disorders, including dementia or seizures, were
excluded from the study.
Surgeries were performed following the same
surgical technique for radical retropubic prostatectomy, as previously
described (11,12). In all surgeries, the residents were assisted by 5
attending surgeons. Fifteen days after hospital discharge, the indwelling
catheter and stitches were removed. The first functional evaluation (urinary
incontinence) was 60 days after surgery, as well as laboratory tests (PSA
value, blood count and serum creatinine).
The length of operative time was measured
from skin incision until the completion of the wound dressing. The estimated
blood loss was calculated by measuring the volume of the vacuum bottle
minus the amount of saline used during surgery. No sponges were used during
surgery.
We also assessed the surgical pathology
stage and Gleason score, in all cases, as well as positive surgical margin
for extracapsular extension. Statistical analysis was performed by using
analysis of variance (ANOVA) and the number of surgeries in quartiles:
up to 10, from 11 to 19, from 20 to 28 and more than 29 surgeries. Fisher’s
exact test was applied to evaluate the groups.
RESULTS
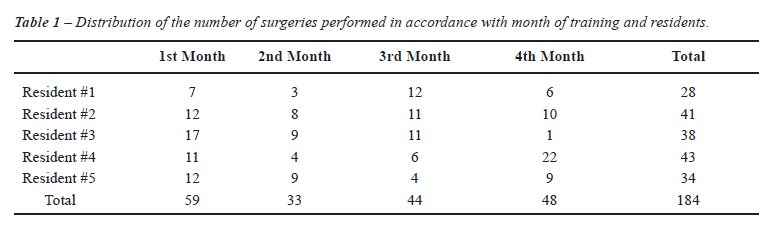
Each resident participated in the study during four consecutive months and, on average, each one of them performed 9 surgeries per month (Table-1).
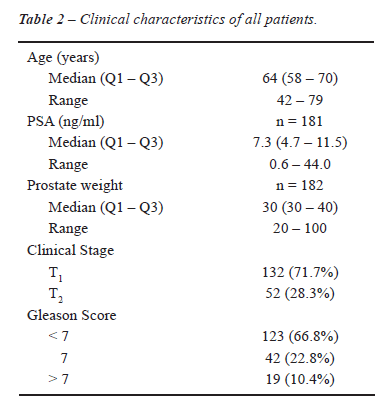
The demographics of patients who underwent
RP are summarized in Table-2.
The surgical pathology stage, prostate size,
Gleason score and surgical margins are summarized in Table-3.
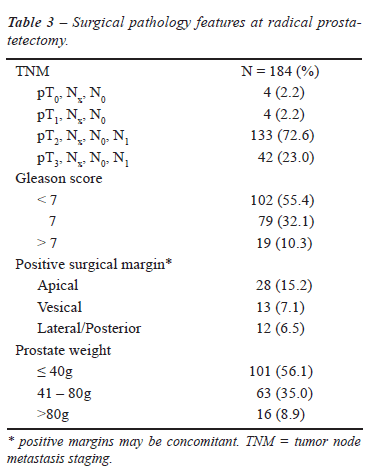
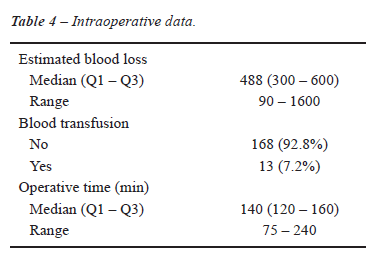
Table-4 presents surgical data. The median
operative time was 140 minutes, and most patients did not require blood
transfusion.
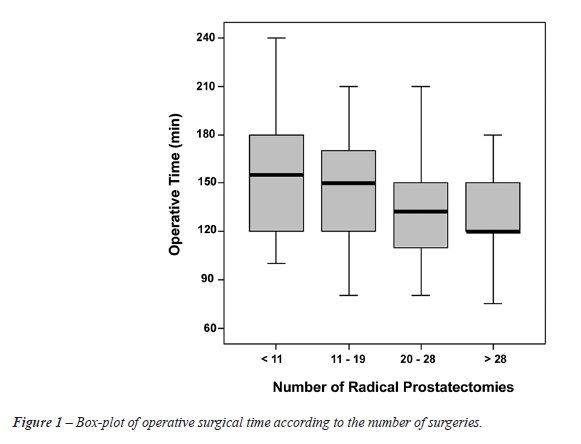
A curve of decreasing operative time (p
= 0.03) is shown in Figure-1, comparing the 19 initial RP to the following
9 RP performed (p = 0.01) and the remaining surgeries from 29 and more
(p < 0.001). From the twentieth RP onwards, we found a significant
decrease in the operative time.
There was a progressive decrease in estimated
blood loss as the residents gained surgical experience with RP, as shown
in Figure-2.
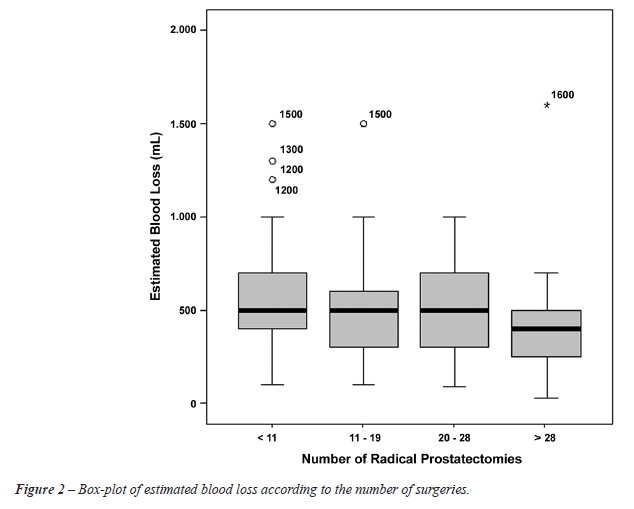
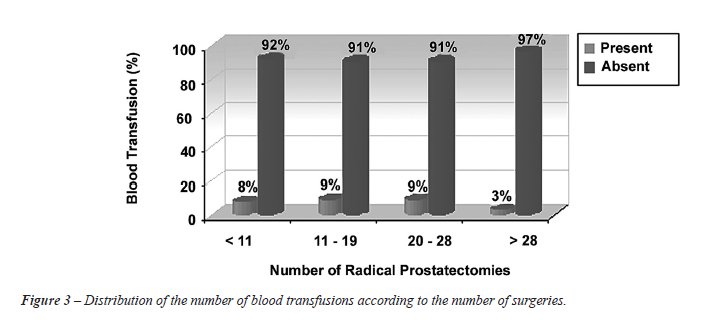
Figure-3 shows the association between the
number of surgeries performed and need for blood transfusion, where a
3% transfusion rate was observed after the 29th surgery.
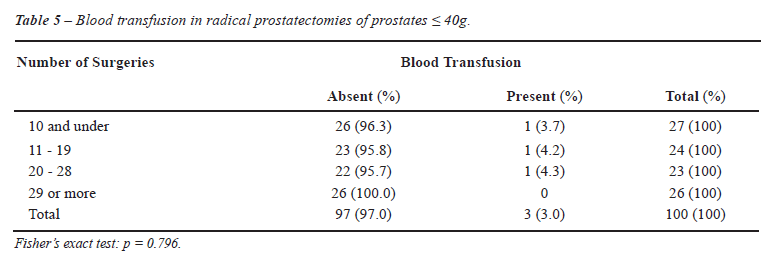
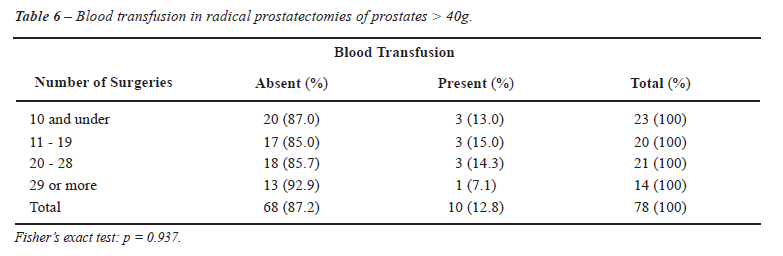
When the resident operated on smaller prostates, blood transfusion was rarely required, as highlighted in Tables 5 and 6, where prostates < 40g and > 40g required a blood transfusion in 3% and 13% of RP, respectively.
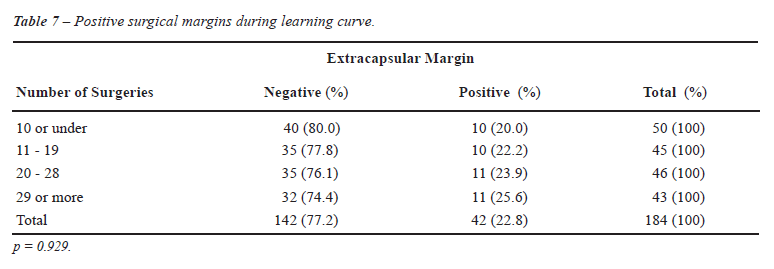
In reviewing the occurrence of positive
surgical margins, we observed that it remained stable during the four
phases, as shown in Table-7.
COMMENTS
The
RP learning curve for residents showed that after twenty surgeries, there
was a significant reduction in operative time from 150 to 120 minutes
and, after the 29th surgery, the need for blood transfusion also decreased
from 9% to 3%. Moreover, the percentage of compromised surgical margins
remained stable during the learning curve.
The discussion regarding the learning curve
in RP has not been frequently addressed in clinical studies and few series
have reported clinical and pathologic data exclusively of residents in
training instead of only experienced surgeons (13,14). Published evidence
has demonstrated that the number of RP previously performed by the surgeon
affects patient outcomes. It is believed that a learning curve of 200
cases would be necessary to achieve an “expert” status (13,15).
A recent prospective study evaluated surgeons
after a urologic oncology fellowship program, after they had already completed
an initial learning curve of an average of 47 cases during residency and
another 87 RP performed during the fellowship (15). The mean operative
time was 201 minutes, the estimated blood loss was 734 mL, with a 6% rate
of blood transfusion.
The learning curve is a major problem in surgery, during which the surgical
procedure is usually performed with more difficulty and slowness, associated
with a higher risk of complications and low efficacy due to inexperience
of the surgeon. If an initial assessment is made, the learning curve is
primarily a theoretical concept, because this is a theme or line of research
rarely present in residency programs and urologic literature.
The surgeons gain much of the knowledge
necessary for surgical procedures during medical residency programs. In
the learning process, the urology resident trains in the areas of endourology,
incontinence and reconstruction, erectile dysfunction and infertility,
pediatric urology and kidney transplantation, laparoscopy and cryotherapy.
Within the urologic oncology division, several surgeries are performed,
such as transurethral resection of the prostate and bladder, cystectomy
and urinary reconstruction, retroperitoneal lymphadenectomy and open and
laparoscopic nephrectomy, fostering a growing field of surgical procedures
and greater confidence to perform them. The American Urological Association
reported that the number of RP performed by residents has declined in
recent years, and overall 84% of surgeons have performed less than ten
RP annually (8). Based on these data, we can infer that much of the surgical
experience needed to acquire proficiency in complex procedures can only
be acquired during residency. Eventually, according to local community
demand or the volume of surgeries performed at the hospital, this development
may never occur.
The percentage of compromised surgical margins
varies with the surgeon’s experience in this procedure. According
to a landmark study by Vickers et al., the rate of positive margins was
36% before 50 RP performed, 29% with 50 to 99 RP, 23% with 100 to 249
RP, 22% with 250 to 999 RP, and 11% with 1000 RP or more (16). Overall,
the surgical margin status was positive in 22.9% of surgeries.
Regarding minimally invasive RP techniques,
usually performed by surgeons in large centers with extensive surgical
experience, data on robotic and laparoscopic was as follows, respectively:
blood transfusion 3% and 9.8%, positive surgical margin of 15.8% and 19.5%,
mean operative time was 166 and 160 minutes, and average hospital stay
of 5.4 and 4.9 days (17). A study describing the learning curve of robotic
RP showed that the robotic surgeon with up to 12 surgeries had an average
operative time of 242 ± 64 minutes and 58% of cases with positive
margins; with 13 to 188 robotic RP, the operative time was reduced to
165 ± 45 minutes and positive margins to 23%. Surgeons who performed
more than 189 robotic RP had an average operative time of 134 ±
45 minutes and positive margins in 9% (18).
The following strengths can be highlighted
in the present study: a homogeneous group of residents in training who
had never performed a RP was included; the prospective design of the study
allowed us to perform the same surgical technique and mentored by the
same group of physician assistants; and the sample size accrued was reasonable.
Therefore, we believe that these results may generate important information
on surgical training and education in urologic oncology.
The fact that the surgeon is inexperienced,
starting the learning curve with this procedure, may be of benefit by
rapidly improving the performance in the short term, considering that
a homogenous and standardized teaching methodology is applied. As our
data suggests, it renders the possibility to generate less intraoperative
morbidity and lower rate of positive surgical margins, improving the clinical
course of patients.
CONCLUSIONS
Open radical prostatectomy is a safe and effective procedure that can be done on a large scale in teaching institutions, as long as a structured training program provides adequate teaching methods. During the initial training experience of a surgeon, a steep reduction in blood transfusions and a quick stabilization of the learning curve after twenty procedures can be expected.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-49.
- Boyle P, Ferlay J: Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005; 16: 481-8.
- Walsh PC, Lepor H, Eggleston JC: Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983; 4: 473-85.
- Elder JS, Jewett HJ, Walsh PC: Radical perineal prostatectomy for clinical stage B2 carcinoma of the prostate. J Urol. 1982; 127: 704-6.
- Guillonneau B, Cathelineau X, Barret E, Rozet F, Vallancien G: Laparoscopic radical prostatectomy: technical and early oncological assessment of 40 operations. Eur Urol. 1999; 36: 14-20.
- Abbou CC, Salomon L, Hoznek A, Antiphon P, Cicco A, Saint F, et al.: Laparoscopic radical prostatectomy: preliminary results. Urology. 2000; 55: 630-4.
- Tewari A, Peabody J, Sarle R, Balakrishnan G, Hemal A, Shrivastava A, et al.: Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology. 2002; 60: 569-72.
- Brasil. Ministério da Saúde: Estimativa 2008: incidência do câncer no Brasil. Instituto Nacional do Câncer. Brasília, DF; 2007.
- Shah A, Okotie OT, Zhao L, Pins MR, Bhalani V, Dalton DP: Pathologic outcomes during the learning curve for robotic-assisted laparoscopic radical prostatectomy. Int Braz J Urol. 2008; 34: 159-62; discussion 163.
- Frota R, Turna B, Barros R, Gill IS: Comparison of radical prostatectomy techniques: open, laparoscopic and robotic assisted. Int Braz J Urol. 2008; 34: 259-68; discussion 268-9.
- Srougi M, Nesrallah LJ, Kauffmann JR, Nesrallah A, Leite KR: Urinary continence and pathological outcome after bladder neck preservation during radical retropubic prostatectomy: a randomized prospective trial. J Urol. 2001; 165: 815-8.
- Srougi M, Paranhos M, Leite KM, Dall’Oglio M, Nesrallah L: The influence of bladder neck mucosal eversion and early urinary extravasation on patient outcome after radical retropubic prostatectomy: a prospective controlled trial. BJU Int. 2005; 95: 757-60.
- Eastham JA, Kattan MW, Riedel E, Begg CB, Wheeler TM, Gerigk C, et al.: Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003; 170: 2292-5.
- Andriole GL, Smith DS, Rao G, Goodnough L, Catalona WJ: Early complications of contemporary anatomical radical retropubic prostatectomy. J Urol. 1994; 152: 1858-60.
- Rosser CJ, Kamat AM, Pendleton J, Robinson TL, Pisters LL, Swanson DA, et al.: Impact of fellowship training on pathologic outcomes and complication rates of radical prostatectomy. Cancer. 2006; 107: 54-9.
- Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al.: The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007; 99: 1171-7.
- Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, et al.: A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol. 2007; 178: 478-82.
- Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, et al.: Robot-assisted laparoscopic prostatectomy: a single-institutions learning curve. Urology. 2009; 73: 127-33.
____________________
Accepted after revision:
May 25, 2010
_______________________
Correspondence address:
Dr. Marcos F. Dall’Oglio
Rua Barata Ribeiro, 398, 5º Andar
01308-000, São Paulo, SP, Brazil
Fax: + 55 11 3159-3618
E-mail: marcosdallogliouro@terra.com.br
EDITORIAL COMMENT
Open radical
prostatectomy is the gold standard and most widespread treatment for clinically
localized prostate cancer.
About thirty years ago the first purposeful nerve sparing radical prostatectomies
were performed by Dr. Patrick Walsh. Since then, a better understanding
of the periprostatic anatomical results with continued improvement in
functional outcomes and oncological control for patients undergoing radical
prostatectomy, whether by open or minimally-invasive surgery.
The oncologic results of author’s paper in an important center of
high volume treatment of prostate cancer are in line with those reported
with the use of the retropubic approach. With a “homogenous and
standardized teaching methodology”, the residents can achieve good
data as regards less intraoperative morbidity and lower rate of positive
surgical margins, improving the clinical course of patients.
The learning curve in surgery can be defined as the number of cases required
to perform the procedure with reasonable operating time and an acceptable
rate of complications, resulting in an adequate postoperative clinical
outcome associated with a shorter hospital stay (1).
A paper was published about the learning curve for surgery for prostate
cancer recurrence after radical prostatectomy. The study cohort included
7765 prostate cancer patients who were treated with radical prostatectomy
by one of 72 surgeons at four major US academic medical centers between
1987 and 2003. The learning curve for prostate cancer recurrence after
radical prostatectomy was steep and did not start to plateau until a surgeon
had completed approximately 250 prior operations (2). As a surgeon’s
experience increases, cancer control after radical prostatectomy improves.
These results may generate important information on surgical training,
improve the teaching process of the surgical technique and make it widely
available to mentors and teaching centers, especially considering the
social environment of growing ethical concerns with patient safety. Further
research is needed to examine the specific techniques used by experienced
surgeons that are associated with improved outcomes.
REFERENCES
- Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al.: The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007; 99: 1171-7.
- Vickers A, Bianco F, Cronin A, Eastham J, Klein E, Kattan M, et al.: The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010; 183: 1360-5.
Dr. Mauricio
Rubinstein
Department of Urology
Federal University of Rio de Janeiro State
Rio de Janeiro, RJ, Brazil
E-mail: mrubins74@hotmail.com
EDITORIAL COMMENT
The learning
curve plateau comes with training and experience. Surgeons have always
recognized a structured way to introduce new procedures: learning a new
technique requires dedication.
If we try to define a learning curve, we should look back at the work
of Dr. Donald Ross - a pioneer in cardiac surgery in the United Kingdom
- who proposed the Ross procedure in 1962 (1). The Ross procedure, first
performed in 1967, is a challenging operation for patients with aortic
valve disease. The principle is to remove the patient’s normal pulmonary
valve and used it to replace the patient’s diseased aortic valve.
In Dr. Ross’s own series, 23% of the patients died during the first
year of the operation and 18% in the second year. The following 10 years,
surgical mortality in a series of 188 patients dropped to 9%. This is
a learning curve. The message: it requires time and hard work.
How many cases do we need to become expert surgeons in the technique we
perform on an everyday basis? The latter remain a controversial question
for the field of radical prostatectomy. The arrival of both, laparoscopy
and later robotic surgery has put on stage the term learning curve. In
fact, laparoscopic series brought with them a tremendous enthusiasm in
terms of validation of the technique and therefore extensive work in the
procedure’s learning curve.
In our experience at the Institut Montsouris in Paris, it was hard to
keep in mind Dr. Walsh’s concepts on radical prostatectomy and simultaneously
comply with the demanding endoscopic surgical environment, but a step-by-step
structured training brought us through the task.
The paper on the learning curve of retropubic radical prostatectomy presented
by Dall’Oglio et al. in this issue of IBJU, represents a comprehensive
analysis of the initial experience of a group of residents with retropubic
prostatectomy, perhaps missing in the literature. The paper offers real
information gained by surgical experience and presents a sincere vision
of a proctored prostatic surgical approach in the everyday world.
Dall’Oglio et al. found in their interesting analysis, that improvement
of clinical outcomes can be seen after 20 to 30 cases. We could say that
these findings are far from those presented by Vickers et al in their
timely publication assessing surgical learning curve for prostate cancer
control (2). Vickers et al. found statistical significance related to
surgeon’s experience and cancer control after radical prostatectomy
in an analysis of highly dedicated surgeons. This study brought back to
reality the definition of learning curve in radical prostatectomy, reflecting
a real link between surgical technique and cancer control, and establishing
the concept of a dramatic improvement in cancer control with increasing
surgeon experience up to 250 previous treated cases. That said, we must
agree with Dr. Stuart Howards in the fact that it is somewhat arbitrary
to assert that it is necessary to perform 250 procedures to become competent
and provide good cancer control (3). Therefore, establishing solid bases
for radical prostatectomy performed in a Urology program, is an important
challenge to any institution and it requires hard dedication and a focused
operating room team; but as presented in the Dall’Oglio et al. study
this is a feasible task and it might get the future urologists ready to
finish their training and be able to offer a surgical procedure of the
highest quality.
The future seems difficult for the young urologist, because as presented
by Ficarra et al., positive surgical margin rates decreased with the surgeon’s
experience and technique improvement, reaching similar percentages for
retropubic, laparoscopic and robotic series (4); but perhaps the positive
surgical margins are not so secure as oncological endpoint (5) and even
our current definitions for biochemical recurrences do not substantially
impact prognostic factor estimates. This situation implies that the training
period should provide solid concepts to build a professional career, and
because knowledge and concepts might and will change; an academic way
to learn, and eventually teach, is the way that it ought to be in order
to assure the adequate surgical treatment of patients, in years to come.
With a structured methodical system, it is possible to implement radical
prostatectomy safely and effectively without compromising morbidity, oncological
and functional outcomes. A team-based approach helps to reduce the learning
curve of the procedure for individual surgeons. This was our initial approach
for laparoscopic and robotic prostatectomy at our institution.
The fruit you harvest from the three in this interesting publication is
that we must be sure to teach the philosophy of how to adequately treat
localized prostate cancer and then, we must get in the operating room
with the urologists-in-training to provide them with the basic tools that
will hopefully sustain future reliable operators.
REFERENCES
- Ross DN: Homograft replacement of the aortic valve. Lancet. 1962; 2: 487.
- Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al.: The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007; 99: 1171-7.
- Howards S: Editorial comment. (Savage et Vickers. Low annual caseloads of United States surgeons conducting radical prostatectomy. J Urol. 2009; 182: 2677-9). J Urol. 2009; 182: 2679-80; discussion 2681.
- Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W: Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol. 2007; 51: 45-55; discussion 56.
- Vickers A, Bianco F, Cronin A, Eastham J, Klein E, Kattan M, et al.: The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010; 183: 1360-5.
Dr. Rafael
Sanchez-Salas and
Dr. Francois Rozet
Department of Urology
Institut Montsouris
Université Paris Descartes
Paris, France
E-mail: francois.rozet@imm.fr
REPLY BY THE AUTHORS
Both editorial
comments reassured the importance of a structured learning methodology
in the surgical field and pointed out the feasibility of safely teaching
complex procedures, such as radical prostatectomy. In our study, we sought
to demonstrate a real learning curve of inexperienced surgeons, on which
we could evaluate both an increasing growth in surgical skills from an
early starting point, combined with the radical prostatectomy training
itself. This was possible due to a homogeneous group of mentors, all of
them with more than 200 prostatectomies performed.
As mentioned in the editorial, the landmark paper by Dr. Vickers demonstrated
a long learning curve, on which improvements were observed to 250 cases
performed (1). This study differed significantly from ours, since not
only no standardized surgical methodology was applied by all 72 surgeons,
but also several very experienced surgeons after urology training were
included in the study.
Although our learning curve demonstrated an initial experience with radical
prostatectomy, the standardized technique had been extensively improved
and validated by Prof. Miguel Srougi, throughout his 4,000 cases (2,3).
Currently, two participating residents in the study are now mentors of
the residents at our institution.
A further study is now being finalized and will focus on the initial 100
consecutive cases operated with this same standardized technique, with
a larger number of surgeons, due to the expansion of our program. Therefore,
we expect to generate a stronger evidence to support the use of our teaching
methodology, which may help to create a gold standard approach for urology
training programs throughout the country.
REFERENCES
- Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al: The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007; 99: 1171-7.
- Srougi M, Nesrallah LJ, Kauffmann JR, Nesrallah A, Leite KR: Urinary continence and pathological outcome after bladder neck preservation during radical retropubic prostatectomy: a randomized prospective trial. J Urol. 2001; 165: 815-8.
- Srougi M, Paranhos M, Leite KM, Dall’Oglio M, Nesrallah L: The influence of bladder neck mucosal eversion and early urinary extravasation on patient outcome after radical retropubic prostatectomy: a prospective controlled trial. BJU Int. 2005; 95: 757-60.
The Authors