RADICAL
CYSTECTOMY WITH PRESERVATION OF SEXUAL FUNCTION AND URINARY CONTINENCE:
DESCRIPTION OF A NEW TECHNIQUE
(
Download pdf )
MIGUEL SROUGI, MARCOS DALL’OGLIO, LUCIANO J. NESRALLAH, HOMERO O. ARRUDA, VALDEMAR ORTIZ
Division of Urology, Paulista School of Medicine, Federal University of São Paulo, UNIFESP, São Paulo, SP, Brazil
ABSTRACT
Objective:
To describe the original cystoprostatectomy technique which allows the
preservation of sexual and urinary function in the majority of treated
patients.
Surgical Technique: The described technique
presents some details that distinguish it from classic cystectomy: 1)
a more efficient control of prostate venous and arterial tributaries;
2) preservation of prostatic capsule and enucleation of prostatic parenchyma,
which is removed in block together with the bladder, without violating
the vesical neck; 3) no manipulation of the distal urethral sphincteric
complex; 4) preservation of seminal vesicles and maintenance of cavernous
neurovascular bundles; 5) wide anastomosis between the ileal neobladder
and the prostatic capsule.
Comments: The proposed maneuvers allow the
performance of radical cystectomy with integral preservation of distal
urethral sphincter and of cavernous neurovascular bundles, without jeopardizing
the oncological principles.
Key
words: bladder; bladder neoplasms; cystectomy; urinary diversion;
urinary reservoirs, continence
Int Braz J Urol. 2003; 29: 336-44
INTRODUCTION
Patients
who have an invasive bladder cancer, stages T2-T4,
are currently treated with radical surgery, radiotherapy, chemotherapy
or with a combination of these approaches (1). According to available
data, radical cystectomy with urinary reconstruction represents the most
effective way for treating such cases, accompanied by cure rates that
oscillate between 53% to 80%, when there is no regional or systemic extension
of the disease (1).
Despite its therapeutic advantages, radical
cystectomy represents a major intervention, accompanied by morbidity rates
that should not be disregarded. In addition to the inherent post-operative
complications, radical cystectomy presented, in the past, 2 serious drawbacks.
Until the final 80s, most of these patients underwent an incontinent cutaneous
urinary diversion, which constrained them to bear urine-collecting bags,
with all the resultant psychological and social drawbacks. Furthermore,
almost all male patients developed erectile dysfunction, which compromised
their quality of life.
Upon the introduction of orthotopic intestinal
neobladders in the urologic practice (2) and the description of the technique
that allowed the preservation of cavernous neurovascular bundles (3),
the drawbacks of cutaneous ostomies and sexual dysfunction were both mitigated,
but they could not be totally avoided. About 10% of patients treated in
this way maintain severe diurnal incontinence and almost half of cases
remain with nocturnal enuresis for extended periods (4). On the other
hand, even when employing the technique for preserving the cavernous bundles,
only 50% of the treated patients evidence penile erections post-operatively
(5).
With the purpose of solving these problems,
Spitz et al. described in 1999 an alternative technique of radical cystectomy
that preserved sexual, ejaculatory, and urinary functions in treated patients
(6). Other studies were subsequently published with the same scope (7,8,9)
and all of them contemplated, in a common way, maneuvers intended to maintain
the integrity of the distal urethral sphincteric complex, responsible
for urinary continence, and the cavernous neurovascular bundles, implied
in the sexual function. Despite the significant reduction in risks of
urinary incontinence and erectile dysfunction, these techniques presented
2 shortcomings witnessed by us in a small number of treated cases. The
block transection of the prostate gland along with the vesical neck is
accompanied by a more marked bleeding that the one observed when employing
classical techniques of radical cystectomy. For the same reason, preservation
of the prostatic parenchyma, common to all such new proposed techniques
creates the risk of incomplete removal of vesical neoplasia, when it infiltrates
and outgrows the vesical neck. For these reasons, we proposed a new technique
of radical cystectomy that aims to preserve the integrity of sexual and
urinary functions and that allows a greater control of intra-operative
bleeding and a more effective resection of tumors located close to the
vesical neck.
SURGICAL TECHNIQUE
With
the patient under general anesthesia, through a wide median abdominal
incision, the bladder is separated from the abdomen anterior wall, maintaining,
together with the organ, the parietal peritoneum that covers it superiorly.
A bilateral pelvic lymphadenectomy is performed, removing the lymph nodes
located around the common, external and internal iliac vessels, and close
to the obturator vessels. Distal ureters in both sides are dissected and
sectioned close to the bladder.
Subsequently, the bladder is laterally released
from the pelvic wall and the vesicoprostatic segment is anteriorly dissected
up to the prostatic apex. The preprostatic fat is removed, carefully controlling
the superficial branch of the deep dorsal vein of penis at the level of
the prostatic apex. The intervention proceeds with transection of lateral
peritoneal wings, which fix the vesical dome to the pelvic wall. Inside
these sheets, we found the vas deferens, and in a more posterior location,
the superior vesical arteries, all of which are sectioned and ligated.
Upon releasing the bladder from the structures
that involve it anteriorly, superiorly and laterally, the hemostatic control
of arterial and venous vessels that involve the prostate begins. Prostatic
arteries, located in the vesico-prostatic sulcus on each side, are ligated
with 2 large and deep “figure-of-8” stitches, with vicryl
zero, applied next to the origin of such vessels in the inferior vesical
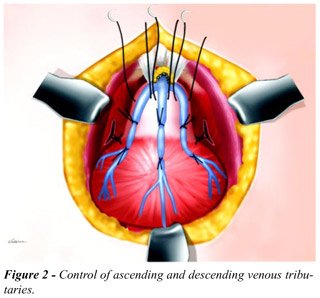
arteries (Figure-1). Next, the 3 venous trunks, one medial and two lateral,
which run over the anterior prostate surface, from the deep dorsal vein
of penis, are controlled. To accomplish this, 2 parallel and transversal
rows of 3 zero vicryl stitches are applied; with the first row located
more distally, at about 1.5 cm from the vesical neck, and other row more
proximally, at 0.5 cm from the neck (Figure-2). These stitches penetrate
deeply the prostate capsule and, once they are tied, they control the
prostate ascending venous tributaries and the bladder descending branches.
They also enable the control of arterial vessels that run in the prostatic
capsule. Before incising the prostatic capsule, a third row of 3 zero
vicryl stitches is done distally to the previous rows, and are not tied
(Figure-2). The anterior portion of the prostatic capsule is incised transversally
with an electrocautery, between the first 2 rows of stitches previously
tied, until the prostatic parenchyma is reached. Through digital and scissors-aided
dissection, the parenchyma is separated from the prostatic capsule, in
a maneuver similar to the one performed when an adenoma is enucleated.
The urethra is sectioned distally, but the base of the prostate is kept
adhered to the vesical neck, forming a single block with the bladder,
whose lumen is not violated (Figure-3). Upon the completion of the distal
enucleation of the prostate, the more distal capsular stitches are tied
and kept repaired. This maneuver allows the definitive control of tributaries
of the deep dorsal vein of the penis, which often start to bleed in the
capsulotomy’s distal margin following the prostatic enucleation.
Such intercurrence results from the loosening of the previously tied capsular
stitches, due to the enucleation of the adenoma.


At this moment, the anterior manipulation
of prostate and bladder is interrupted and the posterior dissection of
the block is proceeded. In order to create a correct plane between the
bladder and the seminal vesicles, which will be preserved, we repaired
the vas deferens in both sides at the posterosuperior surface of the bladder.
Through digital and scissors-aided dissection, the surgeon advances in
caudal direction between the bladder and the vas deferens, and then anteriorly
to the seminal vesicles, until the prostatic base is reached (Figure-4).
Resuming the anterior dissection of the specimen and maintaining a small
sponge between the bladder and the seminal vesicles, the capsulotomy is
completed in its posterior half, with a special precaution to avoid damage
to the cavernous neurovascular bundles (Figure-5). These maneuvers culminate
with the complete release of the bladder-prostatic adenoma block, which
will be removed, and the distal prostatic capsule, preserved. Samples
of tissue from the distal margin of the capsule are removed and submitted
to freezing pathologic study in order to confirm the absence of residual
neoplasia.


Cystectomy is completed sectioning the 2
lateral vesical pedicles, performed with the aid of Mixter forceps or
hemoclamps applied in craniocaudal direction. The specimen formed by the
bladder connected to prostatic adenoma is removed, the prostatic cavity
and the capsular margins are revised and small bleeding vessels are controlled
with electrocautery or with “figure-of-8” 3-zero vicryl stitches.
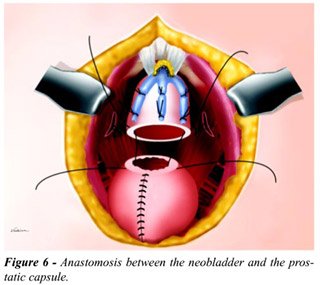
The intervention proceeds with the construction
of an orthotopic ileal neobladder, for which we use Camey II or Studer
techniques (2,10). Once the neobladder is done and double-J catheters
are inserted in both ureters, the anastomosis between the neobladder and
the remaining distal prostatic capsule is performed (Figure-6). This anastomosis
is made with a continuous 2-zero vicryl suture, and before its completion
a 20F Foley urethral catheter is placed in the neobladder, and the distal
ends of both double-J catheters are tied to it.
Surgery is finished with the installation
of continuous suction drains at the level of the anastomosis between the
neobladder and the prostate and near the sites of ureteral implantation.
These drains are maintained until the 7th post-operative day and the Foley
catheter, tied to the double-J catheters, are removed on the 20th day
after the intervention.
COMMENTS
In
this work we present an original alternative technique for radical cystectomy,
which allows integral preservation of urinary continence and reduces substantially
the risks of sexual impotence. In an initial group of 6 treated patients,
5 presented complete diurnal and nocturnal continence immediately after
removing the urethral catheter, 3 referred penile erections on the first
month and none evidenced positive surgical margins at the level of vesical
neck or prostatic parenchyma.
In contrast to the classical radical cystoprostatectomy
technique, this method preserves the prostatic capsule, the cavernous
neurovascular bundles and is not accompanied by manipulation of the distal
urethral sphincteric complex. For such reasons, this technique, which
could be referred to as cysto-adenomectomy, has a highly favorable impact
over the maintenance of urinary and sexual functions post-operatively.
Another advantage of this technique is the fact that it is accompanied
by a block removal of bladder and prostatic parenchyma, reducing the risks
of incomplete removal of the vesical neoplasia, when it invades the vesical
neck and the prostate by intraluminal direct extension.
Under a surgical perspective, this method
incorporates maneuvers that allow a quite efficient control of the anterior
periprostatic venous trunks and the lateral prostatic arteries (11), significantly
reducing intra-operative bleeding. As a matter of fact, none of the 6
patients treated up to now, required blood transfusions during or after
surgery.
The only drawback of the cysto-adenomectomy
technique, compared with the classic radical cystectomy, is that it does
not remove a prostate cancer when this tumor is coincidentally present
in addition to the vesical neoplasia (12). To minimize this problem, the
cysto-adenomectomy technique must be indicated when the existence of a
prostate cancer is highly unlikely, that is, in patients with medical
examination and normal serum levels of prostatic specific antigen or,
in case of doubt, with negative pre-operative prostatic biopsy.
The first proposal about preservation of
sexual and ejaculatory function in radical cystectomy was made in 1999
(6). These authors described a technique that removed the anterior half
of the prostate and preserved its posterior portion. After that, 3 more
studies were published with the same objective, all of them proposing
the preservation of the prostate and bladder resection with distal transection
of the specimen at the level of the vesical neck (7,8,9). Despite highly
elevated rates of maintenance of sexual and urinary function observed
with these techniques, they presented, as a common feature, the risk of
violating the bladder tumor and producing positive distal margins, when
the neoplasia reaches the vesical neck. This risk was reduced by Colombo
et al. (9) and by Vallancien et al. (8) who performed the endoscopic resection
of the vesical neck and the prostate previously, but even then, the potential
risk of violating the neoplasia persists when the vesical neck is sectioned
transversely. In our technique, this possibility is minimized due to the
block removal of prostatic parenchyma, vesical neck and bladder.
Another advantage of this cysto-adenomectomy
technique in relation to other published approaches is that it implies
in removing the specimen in one stage. Both previous endoscopic resection
(9) and that performed at the moment of cystectomy (8) increase the length
and morbidity of the intervention.
If the ongoing study by our group confirms
better preservation of urinary continence and a lower incidence of post-operative
sexual dysfunction, this technique may become a preferential method for
performing radical cystectomy in men with invasive bladder cancer. In
such cases, the method could be employed every time that the presence
of a primary prostate cancer is previously ruled out, and when there is
no extensive secondary involvement of the prostate gland from the vesical
neoplasia.
REFERENCES
- Sternberg CN: Current perspectives in muscle invasive bladder cancer. Eur J Cancer 2002, 38: 460-467.
- Barre PH, Herve JM, Botto H, Camey M: Update on Camey II procedure. World J Urol. 1996, 14: 27-28.
- Schlegel PN, Walsh PC: Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol. 1987, 138: 1402-1406.
- Soulie M, Seguin P, Mouly P, Thoulouzan M, Pontonnier F, Plante P: Assessment of morbidity and functional results in bladder replacement with Hautmann ileal neobladder after radical cystectomy: a clinical experience with highly selected patients. Urology 2001, 58: 707-711.
- Miyao N, Adachi H, Sato Y, Horita H, Takahashi A, Masumori N, et al.: Recovery of sexual function after nerve-sparing radical prostatectomy or cystectomy. Int J Urol. 2001, 8:158-164.
- Spitz A, Stein JP, Lieskovasky G, Skinner DG: Orthotopic urinary, diversion with preservation of erectile and ejaculatory function in man requiring radical cystectomy for nonurothelian malignancy: a new technique. J Urol. 1999, 161: 1761-1764.
- Horenblas S, Meinhardt W, Ijzerman W, Moonen LFM: Sexuality preserving cystectomy and neobladder: initial results. J Urol. 2001, 166: 837-840.
- Vallancien G, El Fettouh HA, Cathelineau X, Baumert H, Fromont G, Guillonneau B: Cystectomy with prostate sparing for bladder cancer in 100 patients: 10-year experience. J Urol. 2002, 168: 2413-2417.
- Colombo R, Bertim R, Salonia A, Da Pozzo LF, Montorsi F, Brausi M, et al.: Nerve and seminal sparing radical cystectomy with orthotopic urinary diversion for selected patients with superficial bladder cancer: an innovative surgical approach. J Urol. 2001, 165: 51-55.
- Modersbacher S, Hechreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al.: Radical cystectomy for bladder cancer today – a homogenous series without neoadjuvant therapy. J Clin Oncol. 2003, 21: 690-696.
- Srougi M, Dall’Oglio MF, Bomfim AC, Andreoni C, Cury J, Ortiz V: An improved technique for bleeding control during simple retropubic prostatectomy. BJU Int (in press).
- Chun TY: Coincidence of bladder and prostate cancer. J Urol. 1977, 157: 65-67.
__________________
Received:
April, 2003
Accepted: May, 2003
_______________________
Correspondence address:
Dr. Miguel Srougi
Rua Peixoto Gomide 2055/81
01409-003, São Paulo, SP, Brazil
Fax: + 55 11 3257-9006
E-mail: srougi@attglobal.net
Srougi
et al. adapted a few technical maneuvers acquired from years of performing
radical and simple prostatectomies and applied them to a cystoprostatectomy
with orthotopic neobladder. The objective is to preserve sexual function
and improve urinary continence. In my experience patients have excellent
daytime continence (< 5% wear pads) although 15% empty by intermittent
catheterization. Nighttime incontinence is infrequent since most of my
patients wake up at least once per night. I have elected to taper the
ileum at the site of the urethral anastomosis which may add to the functional
urethral length. I am not certain how important this is.
The
issue of preserving erectile function is an important one for a relatively
small subset of men who have a cystoprostatectomy and neobladder. The
percentage of men who are candidates for this prostate capsule sparing
is relatively low among all of the men I evaluate for surgery. The majority
of men is older or has advanced local disease and thus they are impotent
or the extent of disease makes invasion of the prostate a concern. I believe
the patient must be one who understands the need for subsequent careful
monitoring - not only for urothelial cancer but for adenocarcinoma of
the prostate.
The
men who are most likely to benefit from these modifications of the standard
cystoprostatectomy are younger men who have recurrent or persistent high
grade Ta, CIS, or T1 bladder cancer and have failed intravesical therapy.
Once tumor at the bladder neck (?) and prostatic urethra is excluded they
might be reasonable candidates for this approach.
There
are some trade-offs when comparing the standard procedure in which the
entire prostate and seminal vesicles are removed with Srougi’s modification
which leaves the prostate capsule and seminal vesicles. This is not much
different however, from leaving the neurovascular bundles, bladder neck,
and the distal seminal vesicles during a radical prostatectomy with the
desire to improve the chance of retaining normal pre-op erectile function.
Each case must be carefully judged based on pre and intraoperative findings
as well as a variety of patient issues such as age and erectile function
before surgery.
______________
Mark S. Soloway
Chairman, Department of Urology
University of Miami School of Medicine
Miami, Florida, USA
EDITORIAL COMMENT
En
bloc removal of the bladder, prostate, ampullae of the vasa deferentia
and seminal vesicles is now the paradigm treatment for muscle invasive
and recurrent high grade urothelial carcinomas. However, largely due to
significant associated morbidities and only modest cure rates when applied
as a monotherapy, initial acceptance of this procedure was not broad.
Over the past 30-years both medical and urologic oncologists have made
dramatic strides to improve the adverse consequences of effectively treating
urothelial cancer. Medical oncologists have graduated patients from non-effective,
single-agent chemotherapy to the latest less-toxic but efficacious combination
of paclitaxel, carboplatin and gemcitabine. With improved survival, urologic
oncologists have modified their surgical execution to reduce morbidity
and improve the social, sexual and psychological implications of radical
cystectomy. Lower urinary tract reconstruction has evolved from simple
cutaneous ureterostomies and ileal conduits to continent cutaneous urinary
reservoirs, and most recently the continent orthotopic neobladders. Today
men and women can safely undergo orthotopic lower urinary tract reconstruction
to the intact native urethra while preserving the erectile nerve bundles
and importantly, the pelvic plexus supplying these nerve bundles. This
has given witness to a dramatic improvements in both the longevity and
quality of our patients’ lives.
For
continuing these forward strides in surgical techniques with their described
method, a modification of that previously described by the USC group (authors’
reference 6), the authors are to be commended. And we are sure that many
innovative surgeons will continue to refine this technique to provide
even better outcomes for the patient of tomorrow.
But
in this search for minimal morbidity, let’s not forget in whom and
for what reason the vast majority of radical cystectomies are performed.
Today the average male patient requiring cystectomy for bladder cancer
is in his sixth decade of life when the reproductive necessity of preserving
ejaculatory function has generally long since past. In the end, this is
the one morbidity of standard nerve sparing radical cystoprostatectomy
that we see as preserved through this technique, and this is accomplished
with a questionable overall improvement in life’s quality for the
vast majority of men with urothelial carcinoma. We are further puzzled
as to the mechanism of antegrade ejaculation following the removal of
a functional bladder neck and the necessity for its coordinated closure
to provide antegrade emission of deposited seminal fluids.
With
a very conscious recognition of the anatomic location of the pelvic plexus
lateral to the seminal vesicles and its nervous supply to the erectile
nerve bundle as depicted in authors’ Figure-1, maintaining the erectile
nerve bundles should be no more difficult during radical cystoprostatectomy
than radical prostatectomy. We commonly perform retrograde release of
the nerve bundles from apex to base during radical cystoprostatectomy
as we perform it during radical prostatectomy. Similar to the reports
of others, this approach has allowed us to preserve erectile function
at rates similar to that seen following isolated radical prostatectomy
(1). While this approach obviates ejaculatory function, is this truly
an issue for the majority of patients undergoing radical cystoprostatectomy?
We
then re-focus on the patients in whom the majority of these surgeries
are performed; sixty-year-old men, who also have the highest incidence
of prostate cancer (2). Furthermore, we now recognize that a significant
number of prostate cancers exist in men with serum PSA below 3ng/ml, the
majority of which are clinically significant (3,4). The benefits of a
radical cystectomy which preserves the posterior lateral zones of the
prostate might quickly fade for the patient and physician alike when the
serum PSA starts rising and there is limited hope of performing completion
prostatectomy and reconstruction of the orthotopic neobladder.
The
authors’ approach is certainly appealing when ejaculatory preservation
is a quality of life issue. We however caution that this select patient
is few and far between. For the young (20 to 30 year-old) male with benign
bladder disease necessitating cystectomy (refractory cystitis glandularis)
or non-urothelial carcinoma away from the bladder neck in whom fertility
is an issue, this technique, offering ejaculatory preservation, even if
ejaculation is retrograde into the neobladder where it can be harvested,
is alluring. For all others we feel there is little benefit to be gained
by this technique over a properly performed nerve sparing cystoprostatectomy
References
- Ghavamian R, Zincke H: An updated, simplified approach to nerve-sparing radical retropubic prostatectomy. BJU Int. 1999; 84:160-163.
- Sarma AV, Schottenfeld D: Prostate cancer incidence, mortality, and survival trends in the United States: 1981-2001. Semin Urol Oncol. 2002; 20:3-9
- Recker F, Kwiatkowski MK, Huber A, Stamm B, Lehmann K, Tscholl R: Prospective detection of clinically relevant prostate cancer in the prostate specific antigen range 1 to 3 ng./ml. combined with free-to-total ratio 20% or less: the Aarau experience. J Urol. 2001; 166: 851-5
- Ward JF, Bartsch T, Sebo TJ, Pinggera G-M, Blute ML, Zincke H: Pathologic characterization of prostate cancers with a very low serum prostate specific antigen (0-2 ng/mL) incidental to cystoprostatectomy: Is PSA a useful indicator of clinical significance? Urol Oncol (in press).
_______________
Dr. John F. Ward
Fellow in Uro-Oncologic Surgery
Department of Urology, Mayo Medical School
Rochester, Minnesota, USA
Dr. Horst
Zincke
Professor of Urology, Mayo Medical School
Consultant, Department of Urology
Rochester, Minnesota, USA
EDITORIAL COMMENT
The
concept of preservation of the prostate at the time of cystectomy for
bladder cancer is not new and has been applied sporadically since early
in the twentieth century. I first preserved the prostate capsule in 1985
using a technique somewhat similar to Srougi and subsequently in several
carefully selected patients. I have been reluctant to publish these cases
due to the uncertainty as to whether this technique will be viable in
the long term. Clearly, interest in this technique has escalated with
this recent report and the larger series of Vallancien et al. from France
(1).
While
there are some early signs that this technique may be beneficial for some
patients, the benefits versus the risks of the technique must be addressed
before its widespread use. The benefits include probable decrease in blood
loss, probable improved continence, and probable improved erectile function
recovery. I emphasize the “probable” aspect because the degree
of improvement is certainly unquantified, despite the initial observations
of Srougi and my experience as well. A validated Quality of Life instrument
suitable to quantify erectile function and incontinence after a cystectomy
and neobladder surgery is not currently available; thus the magnitude
of improvement from this technique will be debatable until studied in
an appropriate fashion.
The
risks are also unquantified. Using whole-mount step-sectioning of the
prostate, it has been determined that approximately 40% of bladder cancer
cystectomy patients may harbor unsuspected urothelial carcinoma in situ
in the prostatic urethra or prostatic ducts (2,3). Similarly, 40% to 50%
of patients have unsuspected adenocarcinoma of the prostate (most of which
are small and of uncertain clinical significance) (3). Since the patient
populations with urothelial and adenocarcinomas do not necessarily overlap,
40% to 80% of patients might have a neoplasm in the prostate which may
make it unwise to leave the prostate capsule behind. Much of this risk
may be obviated by careful patient selection and presurgical screening
for prostate cancers as suggested by Srougi, and a frozen section of the
adenomatous tissue removed from the prostate. If the frozen section reveals
either urothelial or adenocarcinoma at the time of surgery, the prostate
capsule and seminal vesicle could be removed. If the frozen section is
benign, the neobladder could be sewn to the prostate capsule as described
in Srougi’s report.
As
with many procedures in oncology, the evolution is towards smaller, less
extensive operations that still reliably eliminate the cancer but preserve
better function (e.g., lumpectomy and radiation for breast cancer or nerve-sparing
radical prostatectomy). Maybe it is time for a more critical study of
a tailored radical cystectomy for urothelial cancer. Careful patient selection
will unquestionably be the most important aspect.
References
- Vallancien G, Abou El Fettough H, Cathelineau X, Baumert H, Fromont G, Guillonneau B: Cystectomy with prostate sparing for bladder cancer in 100 patients: 10-year experience. J Urol. 2002; 168: 2413-7.
- Wood DP, Montie JE, Pontes JE, VanderBrug Medendorp S, Levin HS: Transitional cell carcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. J Urol. 1989; 141: 346-9.
- Mahadevia PS, Koss LG, Tar IJ: Prostatic involvement in bladder cancer: Prostate mapping in 20 cystoprostatectomy speciems. Cancer 1986; 58: 2096-102.
_________________
Dr. James E. Montie
Chairman, Dept Urology, Univ of Michigan
Valassis Professor of Urologic Oncology
Ann Arbor, Michigan, USA