DEFINITION
AND CURRENT EVALUATION OF SUBFERTILE MEN
(
Download pdf )
SHAI SHEFI, PAUL J. TUREK
Departments of Urology and Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, San Francisco, California, USA
ABSTRACT
Male infertility affects 10% of reproductive aged couples worldwide and is treatable in many cases. In addition to other well-described etiologies, genetic causes of male infertility are now more commonly diagnosed. Using principles of evidence-based medicine, this review outlines diagnostic and treatments options to inform clinical management. In order of importance, randomized controlled clinical trials, basic scientific studies, meta-analyses, case-controlled cohort studies, best-practice policy recommendations and reviews from peer-reviewed literature were incorporated that provide organized and timely guidelines to the current management of male infertility. The strength of evidence for treatment recommendations is also classified when appropriate.
Key
words: male infertility; genetics; semen; spermatozoa; oligospermia;
varicocele
Int Braz J Urol. 2006; 32: 385-97
INTRODUCTION
One
in 6 couples trying to conceive will have difficulty. Infertility is defined
as one year of unprotected intercourse without conception. On evaluation,
roughly 50% of affected couples have causal or associated male factors
as a cause of infertility. In addition, 1-10% of male factor infertility
is a result of an underlying, often treatable, but possibly life-threatening
medical condition (1). For these reasons, the male evaluation is conducted
systematically to acquire relevant information from the history, physical
examination, semen analysis and hormone assessment. Current, evidence-based
diagnostic and treatment algorithms for the management of male infertility
are reviewed in this chapter. The strength of the evidence supporting
a recommendation is graded according to Table-1.

DIAGNOSIS
History
The evaluation of male infertility should
proceed in concert with the female as outlined in Figure-1. A thorough
history is detailed in Table-2 and includes information about not only
medical and surgical problems, but also developmental issues, occupational
and social habits and exposures. Remember that sperm production is very
sensitive to overall body health and problems that make the body ill will
often affect spermatogenesis. The importance of information garnered from
the male infertility history is derived from studies of male physiology
as well as observational studies (Level C evidence).


Physical
Examination
The physical examination assesses body habitus
for obesity, gynecomastia and secondary sex characteristics such as hair
distribution. The phallus may reveal hypospadias, chordee, plaques or
venereal lesions. The testes should be evaluated for their volume, consistency
and contour irregularities suggestive of a mass. Since 80% of testis volume
is determined by spermatogenesis, testis atrophy is likely associated
with decreased sperm production. Palpation of the epididymides might reveal
induration, fullness or nodules indicative of infections or obstruction.
Careful delineation of each vas deferens may reveal agenesis, atresia
or injury. The spermatic cords should be examined for asymmetry suggestive
of a lipoma or varicocele. Clinically significant varicoceles are diagnosed
exclusively by physical examination. Lastly, a rectal examination is important
in identifying large cysts, infections or dilated seminal vesicles.
Semen
Analysis
Although not a true measure of fertility,
the semen analysis, if abnormal, suggests that the probability of achieving
fertility is lower than normal (2). Two semen analyses, performed with
2-3 days of sexual abstinence, are sought due to the large variability
in semen parameters in healthy men (2). Lubricants should be avoided and
the specimen processed during the first hour after ejaculation. There
is recent debate concerning precisely which values are considered “normal”.
The World Health Organization currently recommends 20 million sperm/mL
and 50% motility as normal (2). However, a recent, controlled study of
fertile and infertile couples suggested that a threshold of 48 million
sperm/mL and 63% motility best describes fertile semen (Grade B-C evidence)
(3). Recent data also suggests that spermatogenesis takes < 60 days
to complete instead of 70-80 days as has been thought for 40 years, so
that an individual semen analysis reflects biological influences occurring
2 months prior (4). The formal evaluation of sperm shape is termed morphologic
assessment. Several descriptive systems exist to evaluate morphology.
It is believed that sperm morphology may correlate with a man’s
fertility potential as reflected by in vitro fertilization (IVF) success
(Level C evidence) (5). In general, the percentage of sperm with normal
morphology has the greatest discriminatory power in distinguishing fertile
from infertile semen, although no particular value is diagnostic of fertility
or infertility (3). Sperm morphology complements the routine semen analysis
in the male evaluation and better estimates the chances of fertility.
Hormonal Evaluation
Current recommendations for endocrine evaluation
of the infertile male are: a) sperm concentration < 10 million sperm/mL;
b) erectile dysfunction; c) other clinical signs or symptoms suggestive
of low testosterone or unrelated endocrinopathy. The initial evaluation
should include serum testosterone and follicle stimulating hormone (FSH)
levels. If the testosterone level is low, a repeat testosterone (total
and possibly free testosterone) with luteinizing hormone (LH) and prolactin
serum levels in a morning blood draw is advised. Although an endocrinopathy
is found in 10% of tested men, clinically significant endocrinopathies
are detected in < 2% of men (6).
Genetic
Evaluation
Increasingly, genetic abnormalities are
being identified as causes of male infertility. Thus, genetic testing
should be performed in men with sperm concentrations < 10 million sperm/mL,
with detection of genetic anomalies increasing as sperm concentration
decreases. Deletion of regions on the Y chromosome (microdeletions) occurs
in 6% of men with severely low sperm counts and 13-15% of men with no
sperm counts (7). Deletion of the DAZ (deleted in azoospermia) gene in
the AZFc region is the most commonly observed microdeletion in infertile
men. In addition, 2% of men with low counts and 15-20% of men with no
sperm counts will harbor chromosomal abnormalities detected by cytogenetic
analysis (karyotype). Patients at highest risk for abnormal cytogenetic
findings include men with small, atrophic testes, elevated FSH levels,
and azoospermia. These include conditions such as Klinefelter syndrome
and translocations of non-sex chromosomes. Table-3 outlines current indications
for genetic testing of infertile males (8).
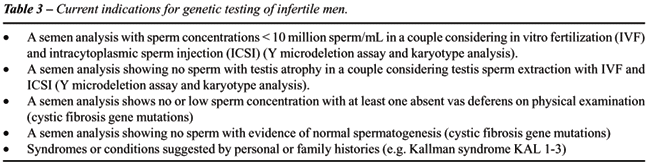
Similarly, genetic testing is indicated
for infertile men who present with cystic fibrosis (CF) or the much more
subtle condition, congenital absence of the vas deferens (CAVD). Similar
genetic mutations are found in both patients, although CAVD patients are
considered to have an atypical form of CF. Approximately 80% of men without
palpable vasa will harbor a CF gene mutation. Recent data also suggest
that azoospermic men with idiopathic obstruction and men with a clinical
triad of chronic sinusitis, bronchiectasis, and obstructive azoospermia
(Young syndrome) may be at higher risk for CF gene mutations.
Other Testing
Anti-Sperm
Antibodies (ASA)
Testing for antisperm antibodies in indicated
if: a) the semen analysis reveals aggregates of sperm; b) there is isolated
asthenospermia; c) there is a risk of autoimmune infertility (i.e. prior
torsion or testis injury); or d) there is unexplained infertility with
a normal routine semen analysis. Usually performed with antibody coated,
polyacrylamide spheres, an ASA test with at least 50% of sperm bound with
antibodies is considered clinically significant. Occurring in 5-10% of
infertile men, it is thought that antibodies bound to the sperm head might
interfere with sperm-egg interaction, penetration and fertilization, whereas
tail bound antibodies may be more likely to affect sperm transport through
the female reproductive tract (9).
Sperm Chromatin Structure Analysis
Recent evidence suggests that the integrity
of sperm DNA-chromatin packaging is important for male fertility. The
structure of sperm chromatin (the DNA-associated proteins) can be measured
by several methods, including the COMET and TUNNEL assays as well as by
flow cytometry after acid treatment and staining of sperm with acridine
orange (10). These tests assess the degree of DNA fragmentation that occurs
after chemically stressing the sperm DNA-chromatin complex, and can indirectly
reflect the quality of sperm DNA integrity. Abnormally fragmented sperm
DNA rarely occurs in fertile men, but can be found in 5% of infertile
men with normal semen analyses and 25% of infertile men with abnormal
semen analyses. This test can detect infertility that is missed on a conventional
semen analysis. Often reversible, causes of DNA fragmentation include
tobacco use, medical disease, hyperthermia, air pollution, infections,
and varicocele. The current indication for this semen assessment is unexplained
infertility.
Post-Ejaculate
Urinalysis (PEU)
To diagnose retrograde ejaculation, the
post-ejaculate urine should be inspected for sperm. A PEU can differentiate
retrograde ejaculation from other causes of low ejaculate volume (<
1.5 mL) including collection error, hypoandrogenism, ejaculatory duct
obstruction, and congenital absence of the vas deferens.
Semen
Leukocyte Evaluation
White blood cells (leukocytes) are present
in all ejaculates and play important roles in immune surveillance and
clearance of abnormal sperm. Leukocytospermia or pyospermia, an increase
in leukocytes in the ejaculate, is defined as > 1 million leukocytes/mL
semen and is a significant cause of male subfertility. The prevalence
of pyospermia ranges from 3% to 23% of infertile men. In general, neutrophils
predominate among inflammatory cells. This condition is detected by a
variety of diagnostic assays, including differential stains (e.g., Papanicolaou),
peroxidase stain that detects the peroxidase enzyme in neutrophils, and
immunocytology. Such testing is indicated with infertility associated
with elevated numbers of “round cells” in the ejaculate on
routine semen analysis.
Ultrasonography
Renal ultrasonography is indicated to evaluate
the possibility of unilateral renal agenesis in CAVD (10% chance with
bilateral vasal agenesis and 25% chance with unilateral vasal agenesis).
Scrotal ultrasound is indicated to evaluate scrotal masses. It is controversial
whether sonography should be used to detect subtle (subclinical) varicocele,
since randomized trials of subclinical varicocele repair reveal no obvious
benefit (Level A evidence). Transrectal ultrasonography (TRUS) is indicated
for low semen volumes to exclude ejaculatory duct obstruction and to evaluate
abnormalities on digital rectal examination.
Vasography
Formal imaging of the reproductive tract
with vasography is warranted in cases of obstructive azoospermia. Vasography
can be undertaken via scrotum, transrectal, transurethral or transperineal
routes. Contrast material can delineate the proximal vas deferens, seminal
vesicle, and ejaculatory duct anatomy, determine whether obstruction is
present and delineate the anatomical site of obstruction. Sampling of
vasal or seminal vesicle fluid during the procedure can also determine
whether sperm exist within these structures, confirming obstruction and
implying the lack of obstruction within the epididymis or testis. Vasography
may also be indicated in the severely oligospermic patient when there
is reason to suspect a unilateral obstruction (such as from a hernia repair)
with an atrophic contralateral testis.
TREATMENT OF MALE INFERTILITY
The
general treatment algorithm for male infertility is outlined in Figure-2.
It is important to ensure that female reproductive potential is adequate
to support the 6-12 month timeline associated with the treatment of most
male factor issues. The decision to select classical treatments for male
infertility including varicocele repair and vasectomy reversal rather
than assisted reproduction is controversial, but accruing evidence from
the urologic literature (Level B-C evidence) suggests that, in most male
factor cases, correcting the male factor is cost-effective. This recommendation
is supported by cost-benefit and decision modeling analyses (11,12). Male
factor infertility can be correctable or uncorrectable, and specifically
treatable or not. These conditions are outlined below.

Correctable
Conditions in the Male
Coital
Timing and Frequency
Easily reviewed and corrected with counseling.
An appropriate frequency for timed intercourse is every 2 days, performed
within the periovulatory period with more emphasis on intercourse prior
to, rather than following, ovulation (Level B evidence) (13). Home kits
that detect the LH surge or the rising estradiol in the urine before ovulation
are generally considered more reliable than basal body temperatures or
untimed sexual intercourse. Coital toxins, including wet heat exposure
from hot tubs or baths, cigarettes, cocaine, marijuana, and excessive
alcohol should be avoided prior to, and during, intended conception.
Abnormalities
of Ejaculation
Significant hypospadias can cause infertility
due imprecise placement of the semen within the cervical os. Surgical
correction or intrauterine insemination (IUI) of sperm are effective treatments.
Severe phimosis, the inability to retract the foreskin, may lead to accumulation
of sperm behind the preputial skin and result in a “low volume”
ejaculate. To improve fertility and hygiene issues, gradual self-dilatation
of the foreskin for cleaning or circumcision are recommended. Erectile
dysfunction (ED) is frequently associated with infertility. Mostly psychogenic
in nature, such cases are effectively treated with sexual counseling and
phosphodiesterase inhibitors. For more severe ED due to organic causes,
medical or surgical treatment is also effective. Retrograde ejaculation
results from a failure of the bladder neck to close during ejaculation.
It can be treated with a trial of sympathomimetic medications. Approximately
30% of men will respond to treatment; those most likely to benefit are
patients with idiopathic causes or diabetes mellitus. The side effects
of these medications usually limit the efficacy of this therapy. For medication
failure, sperm harvesting techniques can be used with IUI to achieve a
pregnancy.
Medications
Are usually tested extensively in animals
for their potential as reproductive hazards before marketing. Despite
this, it is wise to discontinue unnecessary medications that can be safely
stopped during attempts to conceive. A list of gonadotoxic medications
can be found in Table-4. These can result in infertility by various mechanisms.
Ketoconazole, spironolactone, and alcohol inhibit testosterone synthesis,
whereas cimetidine is an androgen antagonist. Recreational drugs such
as marijuana, heroin, and opiates are associated with lower testosterone
levels. Certain pesticides, like dibromochloropropane, are likely to have
estrogen-like activity.

Immunologic
Infertility
It is a complex problem. Several pathological
conditions of the testis, including prior torsion, biopsy, injury or vasectomy,
constitute risk factors for antisperm antibody (ASA) formation. Antibodies
may disturb sperm transport or disrupt normal sperm-egg interaction. Antibodies
may cause clumping or agglutination of sperm, which inhibits passage,
or may block normal sperm binding to the oocyte. Available treatment options
include corticosteroid suppression, sperm washing, intrauterine inseminations
or IVF and ICSI. In general, if > 50% of sperm is bound with antibodies,
then treatment should be offered. Treatment with corticosteroid immunosuppression
should be considered for 6-9 months, and reliably lowers antisperm antibody
levels but might not improve pregnancy rates (Level B evidence) (9). The
risk of aseptic necrosis of the hip makes this treatment option relatively
unattractive to most infertile couples. Intrauterine insemination places
more sperm nearer the ovulated egg to optimize the sperm-egg environment
and can be effective in cases of tail-bound antisperm antibodies. IVF
and ICSI very effectively bypass ASA-related infertility due to either
poor sperm transport through the female reproductive tract, or poor sperm-egg
interaction.
Genital
Tract Infection
The agents most commonly responsible for
male genital tract infections are listed in Table-5. Although genital
tract infection has been linked to infertility in epidemiologic studies,
the correlation between individual organisms and infertility is unclear.
However, various products of activated leukocytes that coexist with genital
infections may impair semen quality. A correlation exists between leukocytes
in semen and the generation of superoxide anions, hydrogen peroxide, and
hydroxyl radicals (reactive oxygen species), all of which can damage sperm
membranes. Sperm are highly susceptible to the effects of oxidative stress
because they possess little cytoplasm and therefore little antioxidant
activity. Damage to sperm from oxidative stress has been correlated to
loss of function and damaged DNA. Given that 83% of all infertile men
will have positive semen cultures and that the relationship between bacterial
cultures and infertility is at best inconclusive, semen cultures should
be obtained only when there are features suggestive of infection, including:
(1) a history of genital tract infection, (2) abnormal expressed prostatic
secretion, (3) the presence of > 1000 pathogenic bacteria/mL semen,
and (4) the presence of > 1 million leukocytes/mL semen (pyospermia).
Uncontrolled studies suggest that pregnancy rates may improve after antibiotic
treatment, but controlled studies do not confirm these findings. Antioxidant
treatment may accompany antibiotics in cases of suspected infection.

Hormonal
Dysfunction
Effective treatment of hormonal disorders
usually involves reversing the specific abnormality detected. Examples
of very correctable conditions include: hyperprolactinemia, hypothyroidism,
congenital adrenal hyperplasia, and testosterone excess or deficiency
due to steroids or genetic conditions like Kallman syndrome. These conditions
should be sought and treated as suggested in Figure-2.
Varicocele
Defined as dilated and tortuous veins within
the pampiniform plexus, the varicocele is the most common surgically correctable
cause of male infertility. Varicocele is a disease of puberty, and is
found in 15% of healthy young men, but in 40% of infertile men. In general,
varicoceles do not spontaneously regress. The cornerstone of varicocele
diagnosis rests on an accurate physical examination. The use of scrotal
ultrasound to detect clinically unapparent (subclinical) varicoceles is
currently unjustified given the lack of treatment efficacy demonstrated
in 3 randomized, controlled trials (Level A evidence) (14). Precisely
how varicoceles exert an effect on the testis remains unclear. Several
theories have been postulated and it is likely that a combination of them
results in infertility. These include: pituitary-gonadal hormonal dysfunction,
internal spermatic vein reflux of renal or adrenal metabolites, an increase
in hydrostatic pressure with venous reflux, and an inhibition of spermatogenesis
through the reflux of warm corporeal blood around the testis and elevation
of intratesticular temperature. Regardless of the biological mechanism,
there is strong evidence to suggest that varicoceles affect semen quality
(15).
The finding of semen abnormalities constitutes
the main indication for varicocele surgery in infertile men. Varicocele
repair should also be considered in adolescents with a large varicocele
and evidence of testis hypotrophy, and in the presence of varicocele-induced
orchialgia. It is important to ensure adequate maternal reproductive potential
if varicocele correction is performed for male infertility, given that
the mean time to natural pregnancy after varicocele surgery is 8 months
(16). In general, it is not advisable to repair varicoceles in with known
genetic infertility, as the chance of improving either semen quality or
natural pregnancy rates is low (17).
In addition to clinical arguments that suggest
varicocele repair benefits infertility, economic analyses also support
this concept. Cost-benefit and decision modeling arguments have shown
that varicocelectomy is more cost effective than ART procedures (11,12).
More recently, it has been demonstrated in “shift of care”
analyses that as many as 50% of couples who would only be candidates for
ART procedures due to low semen quality can be “rescued” from
such procedures and conceive naturally or with IUI after varicocelectomy
(16).
Reproductive
Tract Obstruction
Idiopathic epididymal obstruction is a relatively
uncommon condition found in otherwise healthy men. Although many cases
may result from prior infection, there is recent evidence linking this
condition to cystic fibrosis in that one-third of men may harbor CFTR
gene mutations (18). Inguinal vasal obstruction is an iatrogenic sequel
of the use of knitted polypropylene monofilament mesh prosthesis for inguinal
herniorrhaphy. This “plug and patch” repair method is currently
performed in 80% of inguinal hernia repairs. The post-operative inflammatory
response to mesh can result in fibrosis, which may entrap and obstruct
the inguinal vas deferens. The exact incidence of this problem is currently
undefined (19). Young syndrome presents with a triad of chronic sinusitis,
bronchiectasis, and obstructive azoospermia, with obstruction in the epididymis.
The pathophysiology of the condition is unclear but may involve abnormal
ciliary function or abnormal mucus quality (20). Lastly, obstruction due
to vasectomy is very common in many countries. Reversal of vasectomy by
either vasovasostomy or epididymovasostomy is usually more successful
than when microsurgery performed to correct any of the other conditions
listed above, and is highly dependent on the skill and technique of the
surgeon.
Ejaculatory duct obstruction (EDO) is a
unique form of blockage within the male reproductive tract. It presents
with low volume azoospermia (complete obstruction) or low volume oligoasthenospermia
(partial obstruction). Obstruction can be congenital and result from Müllerian
duct (utricular) cysts, Wolffian duct (diverticular) cysts, or atresia,
or can be acquired from seminal vesicle calculi or postsurgical or inflammatory
scar tissue. Based on a suspicious semen analysis, transrectal ultrasound
(TRUS) is performed to confirm the diagnosis. Recent literature suggests
that: a) TRUS, as a static test, can over-diagnose EDO in up to 50% of
cases as some “obstruction” may be functional and not physical,
b) the addition of “dynamic” imaging prior to surgical treatment,
such as ejaculatory duct chromotubation or vasography, can distinguish
true obstruction from functional obstruction, and c) partial obstruction
is more difficult to diagnose than complete obstruction as TRUS findings
are less specific for partial obstruction (21). Overall, a 20-30% pregnancy
rate can be expected from endoscopic treatment of EDO, and 70-80% of men
will achieve a significant improvement in semen quality. Complications
occur in 10% of cases and include hematuria, watery ejaculate and epididymitis.
Uncorrectable Conditions in the Male
Chemotherapy
and Radiotherapy
Chemotherapy is designed to kill rapidly
dividing malignant or diseased cells; an undesired outcome is the cytotoxic
effect on normal tissues. Differentiating spermatogonia appear to be the
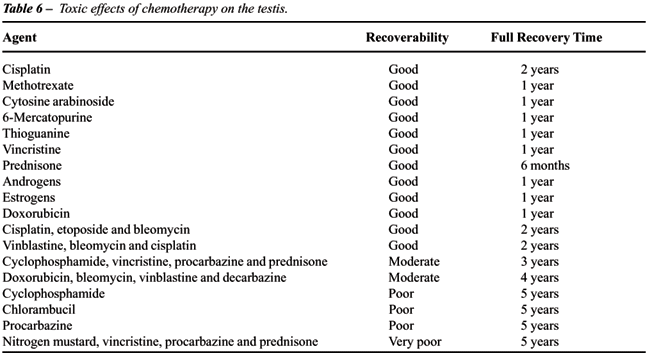
germ cells most sensitive to chemotherapy. Alkylating agents such as cyclophosphamide,
chlorambucil, and nitrogen mustard are the most toxic agents. A list of
agents and their relative toxicity to the testis (from decreasing to increasing
effect) is given in Table-6. The toxicity of chemotherapy varies widely
according to dose and duration of treatment, type and stage of disease,
age and health of the patient, and baseline testis function. Despite this
toxicity, the mutagenic effects of chemotherapy do not appear to be significant
enough to increase the chance of congenital defects or genetically linked
diseases among naturally conceived offspring. However, patients should
wait at least 6 months after chemotherapy ends before attempting to conceive.
Men with continued azoospermia after treatment are very likely (65%) to
have testis sperm available for use with IVF and ICSI (22).
The effects of radiotherapy on sperm production
are well described and derived mainly from a series of experiments performed
on healthy prisoners in Oregon and Washington in the 1960s (23). From
these studies (Level B evidence), a significant reduction in sperm count
is observed at 15 cGy exposure, and sperm counts are temporarily abolished
at 50 cGy. Persistent azoospermia is induced at 400 cGy. Recovery from
radiation exposure can take up to 1 year. Similar to chemotherapy, there
does not appear to be an increase in congenital birth defects in naturally
conceived offspring of irradiated men.
Congenital
or Acquired Obstruction
Cystic fibrosis (CF) is the most common
autosomal recessive genetic disorder in the United States. It is caused
by CFTR mutations and is fatal. It is associated with fluid and electrolyte
abnormalities (abnormal chloride-sweat test) and presents with chronic
lung obstruction and infections, pancreatic insufficiency, and infertility.
Interestingly, 99% of men with CF have mesonephric duct abnormalities,
including absent of atrophic vas deferens, seminal vesicles, and ejaculatory
ducts. Spermatogenesis is usually normal. Another group of men without
obvious signs of CF may also exhibit reproductive tract abnormalities
that are unreconstructable (congenital absence of the vas deferens, CAVD).
This is a form of CF, since 80% of these patients will harbor a detectable
CF mutation and 15% will have renal malformations, most commonly unilateral
agenesis (24). In either case, sperm retrieval from the testis or epididymis
is effective in most cases (25). Genetic counseling the testing in these
patients is important to define the residual risk of CF disease transmission
to offspring (26).
Adult polycystic kidney disease is an autosomal
dominant disorder associated with numerous cysts of the kidney, liver,
spleen, pancreas, epididymis, seminal vesicle, and testis. Disease onset
usually occurs in the twenties or thirties with symptoms of abdominal
pain, hypertension, and renal failure. Infertility with this disease is
usually secondary to obstructing cysts in the epididymis or seminal vesicle.
Functional blockages may result from nerve injury or medications that
impair the contractility of seminal vesicle or vasal musculature. A classic
example is nerve injury after retroperitoneal lymph node dissection for
testis cancer, causing either retrograde ejaculation or complete anejaculation,
depending on the degree of injury to postganglionic sympathetic fibers.
Multiple sclerosis, spinal cord injury and diabetes are other conditions
that result in disordered ejaculation. Some forms of anejaculation are
treatable with rectal probe electroejaculation or penile vibratory stimulation
along with intrauterine insemination; others are currently not correctable
and require higher forms of assisted reproduction for fatherhood (27).
Genetic
Male Infertility
Overall, 50% of male infertility is currently
unexplained and it is likely that much of this is has a genetic basis.
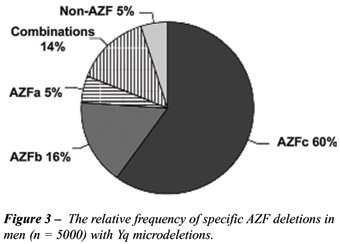
Over the last decade, a strong association has arisen between deletions
in several regions of the Y chromosome and male infertility. They are
termed the Azoospermia Factor regions AZFa, AZFb, AZFc. The relative frequency
of individual AZF deletions is illustrated in Figure-3 (7). Another group
of men with oligospermia (2%) or azoospermia (15%) will harbor abnormalities
in chromosomal number or constitution as assessed by a karyotype analysis.
In general, affected men present with testis atrophy and elevated follicle
stimulating hormone levels, similar to non-genetic infertility. In the
presence of genetic infertility, there is no evidence that classical medical
or surgical treatments for male infertility will be successful (17). Instead,
affected patients should proceed to assisted reproduction with either
ejaculated or surgically retrieved sperm.
One large obstacle to biological family
building in men with genetic infertility and azoospermia is the fact that
only 50-60% of men with this condition will harbor usable sperm in the
testis form IVF-ICSI. This state of affairs is also true in men with nonobstructive
azoospermia in which a genetic etiology is not formally defined, but only
suspected. Unfortunately, clinical features of testicular size, history
of ejaculated sperm, serum FSH level, or biopsy histology do not accurately
predict whether or not sperm will be recovered on exploration. Because
of this, strategies have been developed to more accurately determine which
men with failing testes are candidates for IVF-ICSI, and surgical techniques
have been refined to minimize the invasiveness of sperm harvest procedures.
One strategy involves taking as many biopsy samples as needed either before
IVF-ICSI (and freezing the sperm for later thaw and use), or at the time
of ICSI (28). Microdissection TESE uses microsurgical exploration of the
widely opened testis to search for pockets of sperm (29). Finally, diagnostic
testis fine needle aspiration (FNA) “mapping” seeks to determine
patient candidacy for future sperm testis retrieval (30). Subsequently,
at ICSI, needles or biopsies are “directed” to testis locations
informed by the map. Armed with the knowledge of sperm presence from prior
“mapping” we have found that the need for testis microdissection
during sperm retrieval can be reduced to roughly 20% of cases, with the
vast majority requiring less invasive methods to retrieve sperm, including
TESA (testis sperm aspiration by needle) or TESE (testis sperm extraction
by small biopsy). A review of 8 single institution studies using either
microdissection or FNA mapping suggests that about 50% of nonobstructive
azoospermic men with have sperm found by either technique.
Over the last decade, several interesting
observations have been made regarding genetic infertility and the potential
to father children. It appears that the presence or absence of sperm (ejaculated
or retrieved) in men with Y chromosome microdeletions varies depending
on the specific deletion. In contrast to partial and complete AZFc deletion
patients, in whom sperm can be found on semen analysis or in the testis
70% of the time, the chance of finding sperm in men with complete AZFa
or AZFb deletions is unlikely (31). However, since fewer than 100 patients
with isolated AZFa or AZFb deletions have been reported, more patients
are needed to confirm this statement. In addition, from four studies that
have addressed the IVF-ICSI outcomes in men with Y deletions (all AZFc
deleted patients), it appears that fertilization rates, embryo quality
and pregnancy rates approximate non Y-deleted contemporary controls. Furthermore,
it has been shown that men with AZF deletions who conceive with IVF-ICSI
will pass on the Yq deletion to male offspring despite being somatically
healthy. Lastly, there has been an observation that there may be a time-dependent
decline in sperm production in Yq deleted patients, which leads some to
suggest that affected patients should consider cryopreservation of ejaculated
sperm in early adulthood (32).
CONFLICT OF INTEREST
None declared.
REFERENCES
- Honig SC, Lipshultz LI, Jarow JP: Significant medical pathology uncovered by a comprehensive male infertility evaluation. Fert Steril. 1994; 62: 1028-34.
- WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction, Fourth edition. Cambridge, Cambridge University Press. 1999; pp. 23-25.
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al.: Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001; 345: 1388-93.
- Misell LM, Holochwost D, Boban D, Santi N, Shefi S, Hellerstein MK, et al.: A stable isotope-mass spectrometric method for measuring human spermatogenesis kinetics in vivo. J Urol. 2006; 175: 242-6; discussion 246.
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S: Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988; 49: 112-7.
- Sigman M, Jarow JP: Endocrine evaluation of infertile men. Urology. 1997; 50: 659-64.
- Foresta C, Moro E, Ferlin A: Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001; 22: 226-39.
- Turek PJ: Practical approach to the diagnosis and management of male infertility. Nature Clin Pract Urol. 2005; 2: 1-13.
- Turek PJ, Lipshultz LI: Immunologic infertility. Urol Clin North Am. 1994; 21: 447-68.
- Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al.: Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999; 14: 1039-49.
- Meng MV, Greene KL, Turek PJ: Surgery or assisted reproduction? A decision analysis of treatment costs in male infertility. J Urol. 2005; 174: 1926-31; discussion 1931.
- Schlegel PN: Is assisted reproduction the optimal treatment for varicocele-associated male infertility? A cost-effectiveness analysis. Urology. 1997; 49: 83-90.
- Wilcox AJ, Weinberg CR, Baird DD: Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995; 333: 1517-21.
- Evers JL, Collins JA: Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003; 361: 1849-52.
- No authors listed: The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril. 1992; 57: 1289-93.
- Cayan S, Erdemir F, Ozbey I, Turek PJ, Kadioglu A, Tellaloglu S: Can varicocelectomy significantly change the way couples use assisted reproductive technologies? J Urol. 2002; 167: 1749-52.
- Cayan S, Lee D, Black LD, Reijo Pera RA, Turek PJ: Response to varicocelectomy in oligospermic men with and without defined genetic infertility. Urology. 2001; 57: 530-5.
- Jarvi K, Zielenski J, Wilschanski M, Durie P, Buckspan M, Tullis E, et al.: Cystic fibrosis transmembrane conductance regulator and obstructive azoospermia. Lancet. 1995; 345: 1578.
- Shin D, Lipshultz LI, Goldstein M, Barme GA, Fuchs EF, Nagler HM, et al.: Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia. Ann Surg. 2005; 241: 553-8.
- Handelsman DJ, Conway AJ, Boylan LM, Turtle JR: Young’s syndrome. Obstructive azoospermia and chronic sinopulmonary infections. N Engl J Med. 1984; 310: 3-9.
- Purohit RS, Wu DS, Shinohara K, Turek PJ: A prospective comparison of 3 diagnostic methods to evaluate ejaculatory duct obstruction. J Urol. 2004; 171: 232-5; discussion 235-6.
- Damani MN, Master V, Meng MV, Burgess C, Turek P, Oates RD, et al.: Postchemotherapy ejaculatory azoospermia: fatherhood with sperm from testis tissue with intracytoplasmic sperm injection. J Clin Oncol 2002; 20: 930-6.
- Clifton DK, Bremner WJ: The effect of testicular x-irradiation on spermatogenesis in man. A comparison with the mouse. J Androl. 1983; 4: 387-92.
- Chillon M, Casals T, Mercier B, Bassas L, Lissens W, Silber S, et al.: Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995; 332: 1475-80.
- Nudell DM, Conaghan J, Pedersen RA, Givens CR, Schriock ED, Turek PJ: The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod. 1998; 13: 1260-5.
- Danziger KL, Black LD, Keiles SB, Kammesheidt A, Turek PJ: Improved detection of cystic fibrosis mutations in infertility patients with DNA sequence analysis. Hum Reprod. 2004; 19: 540-6.
- Master VA, Turek PJ: Ejaculatory physiology and dysfunction. Urol Clin North Am. 2001; 28: 363-75.
- Mulhall JP, Burgess CM, Cunningham D, Carson R, Harris D, Oates RD: Presence of mature sperm in testicular parenchyma of men with nonobstructive azoospermia: prevalence and predictive factors. Urology. 1997; 49: 91-5; discussion 95-6.
- Schlegel PN: Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999; 14: 131-5.
- Turek PJ, Ljung BM, Cha I, Conaghan J: Diagnostic findings from testis fine needle aspiration mapping in obstructed and nonobstructed azoospermic men. J Urol. 2000; 163: 1709-16.
- Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN: Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003; 18: 1660-5.
- Krausz C, Bussani-Mastellone C, Granchi S, McElreavey K, Scarselli G, Forti G: Screening for microdeletions of Y chromosome genes in patients undergoing intracytoplasmic sperm injection. Hum Reprod. 1999; 14: 1717-21.
________
Accepted:
February 3, 2006
_______________________
Correspondence address:
Dr. Paul J. Turek
Endowed Chair in Urologic Education
Department of Urology - UCSF
1600 Divisadero St, Rm. A633
San Francisco, CA 94143-1695
Fax: + 1 415 885-7443
E-mail: pturek@urology.ucsf.edu