RE:
INFLAMMATORY ATROPHY ON PROSTATE NEEDLE BIOPSIES: IS THERE TOPOGRAPHIC
RELATIONSHIP TO CANCER?
(
Download pdf )
ATHANASE BILLIS, LEANDRO L.L.FREITAS, LUIS A. MAGNA, UBIRAJARA FERREIRA
Departments of Anatomic Pathology (AB,LLLF), Genetics and Biostatistics (LAM), and Urology (UF), School of Medicine, State University of Campinas (Unicamp), Campinas, São Paulo, Brazil
Int Braz J Urol 2007;33:355-63
To the Editor:
The
editorial comments of our paper by Dr.H.Samaratunga, Dr. Rodolfo Montironi,
and Dr. Liang Cheng were very informative on a lesion that is one of the
most frequent mimics of prostatic adenocarcinoma. It occurs most frequently
in the posterior lobe or peripheral zone (1-3) and gained importance with
the increasing use of needle biopsies for the detection of prostatic carcinoma
(4). Moore (1), in 1936, was one of the first authors to describe prostatic
atrophy in a systematic autopsy study. He found that there was a strong
correlation with age and, according to his study, prostatic atrophy is
initiated during the 5th decade and continues as a progressive process
into the 8th decade. It is a frequent lesion: 85% in autopsies and 83.7%
in needle biopsies (5,6).
Why this lesion mimics adenocarcinoma? Histologically
prostatic atrophy may be partial or complete. The latter is subtyped in
simple, hyperplastic (or post-atrophic hyperplasia), and sclerotic (5).
It seems that the subtypes represent a morphologic continuum of a single
lesion (4). Partial atrophy and hyperplastic (or postatrophic hyperplasia)
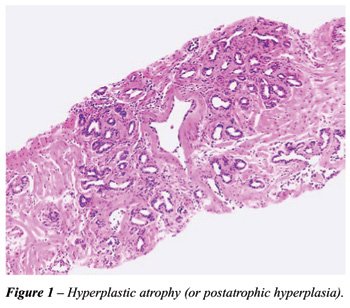
most frequently mimic adenocarcinoma. Hyperplastic atrophy shows small
acini closely packed together and lined by atrophic epithelium. Fibrosis
is present or not in the stroma. When present, the proliferation is irregular
and can result in distortion of the acini simulating stromal infiltration
(Figure-1). Partial atrophy was described by Oppenheimer et al. (7). The
name is due to the fact that there is partial preservation of the cytoplasm
simulating neoplastic micro-acini (Figure-2). An additional pitfall for
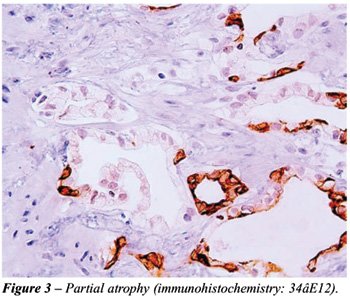
the surgical pathologist is the fact that in partial atrophy the basal
cells may be scattered and in some acini may be completely absent (Figure-3).

There are some findings associated to the
etiopathogenesis of the lesion. Atrophy is clearly associated to advanced
age (1,5). Radiotherapy and hormonal deprivation are associated with diffuse
atrophy. Inactive or active inflammation is a frequent cause for the lesion
(8) and based on a study on autopsies there is evidence that chronic local
ischemia may also be a cause of atrophy (5). However, many examples of
atrophy are still considered idiopathic in nature. Both inflammation and
ischemia are associated with focal forms of atrophy.
The relation of prostatic atrophy to neoplasia
is exciting and controversial. This topic was thoroughly commented in
our study and discussed in the editorial comments by Dr.H.Samaratunga,
Dr. Rodolfo Montironi, and Dr. Liang Cheng (6).
In diagnostic practice it is not rare to
find patients with serum prostate- specific antigen (PSA) elevation and
several biopsies showing no atypical, preneoplastic or neoplastic lesions,
except prostatic atrophy. Regardless of the cause, we hypothesized that
damaged epithelial cells in atrophic acini could be a source of the elevation
of PSA. Our study was based on 131 needle prostatic biopsies corresponding
to 107 patients. The only diagnosis in all biopsies was focal prostatic
atrophy without the presence of cancer, high-grade prostatic intraepithelial
neoplasia, or areas suspicious for cancer. A positive and significant
association was found between the extent of atrophy and the total or free
serum PSA elevation (9). All patients showing 35mm or higher linear extent
of atrophy in the biopsy cores, had serum PSA above 4ng/mL. The findings
suggest that damaged epithelial cells in atrophic acini, regardless of
cause, could be a source of serum PSA elevation.
Prostate-specific antigen is a single chain
glycoprotein with proteolytic enzyme activity mainly directed against
the major gel-forming protein of the ejaculate (semenogelin). PSA induces
liquefaction of semen with release of progressively motile spermatozoa
(10). There are several efficient physiologic barriers to prevent the
escape of any significant amounts of PSA from the prostatic ductal system:
basement membrane of the acini, basal cells lining the acini, prostatic
stroma, basement membrane of capillary endothelial cells, and endothelial
cells. These barriers normally prevent PSA from entering the general circulation
at concentrations of more than 3ng/mL (10).
Focal prostatic atrophy represents a form
of adaptive response to injury most commonly to inflammation and/or local
ischemia. It is intriguing that atrophic acini may produce an excess of
serum PSA. Inflammation and/or ischemia are injurious stimuli resulting
in diminished oxidative phosphorilation, membrane damage, influx of intracellular
calcium, and accumulation of oxygen-derived free radicals (oxidative stress)
(11). We speculate that these injurious stimuli may interfere in the physiologic
barrier that prevent the escape of any significant amounts of PSA to the
general circulation.
Atrophy is a frequent, exciting, intriguing
lesion and a relevant subject for further research. Pathologists should
include the presence and extent of the lesion in the pathology report.
References
1. Moore RA: The evolution and involution of the prostate gland. Am J
Pathol. 1936;12:599-624.
2. Franks LM: Atrophy and hyperplasia in the prostate proper. J Pathol
Bacteriol. 1954;68:617-21.
3. Liavag I: Atrophy and regeneration in the pathogenesis of prostatic
carcinoma. Acta Pathol Microbiol Scand [A]. 1968;73:338-50.
4. Cheville JC, Bostwick DG: Postatrophic hyperplasia of the prostate.
A histologic mimic of prostatic adenocarcinoma. Am J Surg Pathol. 1995;19:1068-76.
5. Billis A: Prostatic atrophy: An autopsy study of a histologic mimic
of adenocarcinoma. Mod Pathol. 1998;11:47-54.
6. Billis A, Leandro LL Freitas, Luis A Magna, Ubirajara Ferreira: Inflammatory
atrophy on prostate needle biopsies: Is there topographic relationship
to cancer? Int Braz J Urol. 2007;33:355-63.
7. Oppenheimer JR, Wills ML, Epstein JI: Partial atrophy in prostate needle
cores: another diagnostic pitfall for the surgical pathologist. Am J Surg
Pathol. 1998;22:440-5.
8. Srigley JR: Benign mimickers of prostate cancer. Mod Pathol. 2004;17:328-48.
9. Billis A, Meirelles LR, Magna LA, Baracat J, Prando A, Ferreira U.
Extent of prostatic atrophy in needle biopsies and serum PSA levels: is
there an association? Urology. 2007;69:927-30.
10. Oesterling JE, Lilja H: Prostate-specific antigen. The value of molecular
forms and age-specific reference ranges. In: Vogelzang NJ, Scardino PT,
Shipley WU, Coffey DS (eds.), Comprehensive textbook of genitourinary
oncology. Baltimore, Williams & Wilkins. 1996; pp.668-80.
11. Kumar V, Abbas AK, Fausto N: Robbins and Cotran Pathologic Basis of
Disease, 7th ed. Philadelphia, Elsevier Sanders.2005; pp.3-46.
Dr. Athanase Billis
Full-Professor of Pathology
State University of Campinas, Unicamp
Campinas, São Paulo, Brazil
E-mail: athanase@fcm.unicamp.br