SIMULTANEOUS
LAPAROSCOPIC NEPHROURETERECTOMY AND CYSTECTOMY: A PRELIMINARY REPORT
(
Download pdf )
RODRIGO BARROS, RODRIGO FROTA, ROBERT J. STEIN , BURAK TURNA, INDERBIR S. GILL, MIHIR M. DESAI
Section of Laparoscopic and Robotic Surgery, Glickman Urological Institute, Cleveland Clinic Foundation, Cleveland, Ohio, USA
ABSTRACT
Purpose:
Patients with muscle-invasive bladder cancer and concomitant upper urinary
tract tumors may be candidates for simultaneous cystectomy and nephroureterectomy.
Other clinical conditions such as dialysis-dependent end-stage renal disease
and non-functioning kidney are also indications for simultaneous removal
of the bladder and kidney. In the present study, we report our laparoscopic
experience with simultaneous laparoscopic radical cystectomy (LRC) and
nephroureterectomy.
Materials and Methods: Between August 2000
and June 2007, 8 patients underwent simultaneous laparoscopic radical
nephroureterectomy (LNU) (unilateral-6, bilateral-2) and radical cystectomy
at our institution. Demographic data, pathologic features, surgical technique
and outcomes were retrospectively analyzed.
Results: The laparoscopic approach was technically
successful in all 8 cases (7 males and 1 female) without the need for
open conversion. Median total operative time, including LNU, LRC, pelvic
lymphadenectomy and urinary diversion, was 9 hours (range 8-12). Median
estimated blood loss and hospital stay were 755 mL (range 300-2000) and
7.5 days (range 4-90), respectively. There were no intraoperative complications
but only 1 major and 2 minor postoperative complications. The overall
and cancer specific survival rates were 37.5% and 87.5% respectively at
a median follow-up of 9 months (range 1-45).
Conclusions: Laparoscopic nephroureterectomy
with concomitant cystectomy is technically feasible. Greater number of
patients with a longer follow-up is required to confirm our results.
Key
words: kidney; ureter; laparoscopy; nephrectomy; cystectomy;
TCC
Int Braz J Urol. 2008; 34: 413-21
INTRODUCTION
Transitional
cell carcinoma (TCC) of the bladder is the sixth most common malignancy
in the United States, accounting for 10% of cancers in men and 4% in women
(1). While open radical cystectomy (ORC) and urinary diversion remain
the gold standard for treatment of muscle-invasive TCC of the bladder,
laparoscopic radical cystectomy (LRC) has been gaining popularity and
presently the worldwide experience includes more than 500 cases (2). Treatment
of bladder tumor may be complicated with concurrent upper tract disease.
Palou et al. have reported a 1.8% incidence of simultaneous upper tract
and bladder TCC, where 46% of the bladder tumors were found to be invasive
(3).
Radical nephroureterectomy with bladder
cuff excision is considered the standard of care for high-grade, invasive,
recurrent, or large volume TCC of the upper urinary tract (UUT). Since
the first description of laparoscopic nephroureterectomy (LNU) by Clayman
et al. (4), several authors have demonstrated improved recovery with equivalent
intermediate-term oncologic outcomes using the laparoscopic approach compared
to open radical nephroureterectomy (5-8).
In patients with recurrent high grade or
muscle invasive bladder TCC and concomitant UUT tumors, simultaneous cystectomy
and nephroureterectomy is the principle oncologic procedure of choice
(9-11). Other benign clinical conditions including dialysis-dependent
end-stage renal disease (ESRD) or non-functioning kidney are relative
indications for simultaneous upper unilateral or bilateral nephroureterectomy
and lower tract extirpation (9). The aim of this report is to describe
our experience with combined laparoscopic radical cystectomy and nephrouretererectomy.
MATERIALS AND METHODS
Between
August 2000 and June 2007, 8 patients underwent simultaneous laparoscopic
radical cystectomy and nephroureterectomy at our institution. All procedures
were performed by the same surgical team. Demographic data and pathologic
features of the bladder and upper tract tumors were individually recorded.
Perioperative outcomes, postoperative pathologic data and oncologic outcomes
were retrospectively reviewed and analyzed.
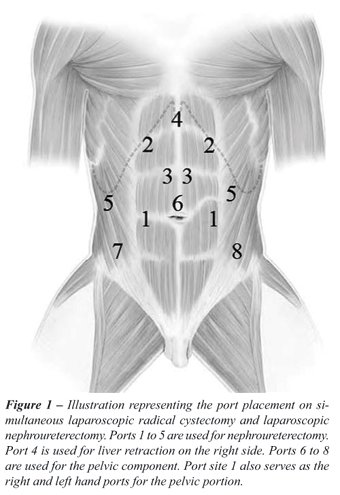
Our surgical technique for the laparoscopic
procedure is as follows. Initially, the patient is placed in 60-degree
flank position for transperitoneal radical nephroureterectomy. Port placement
is depicted in Figure-1 for nephroureterectomy and cystectomy. Notably,
the primary port (12 mm) is inserted at the site of the proposed ileal
conduit stoma for a right-sided nephroureterectomy or at the edge of the
rectus muscle along a line between the umbilicus and anterior-superior
iliac spine for a left-sided nephroureterectomy. During right-sided nephroureterectomy,
this port will serve as the left hand port during the upper tract procedure
and right hand port during the cystectomy. Conversely, for left-sided
nephroureterectomy this port serves as the right hand port for the upper
tract procedure and left hand port for the pelvic portion. On the right
side, ports 2-5 are placed as for our standard nephroureterectomy. Notably,
port 4 is used for an Allis clamp locked to the side wall as a self-retaining
liver retractor and an instrument placed through port 5 is used for lateral
retraction. Left-sided port placement mirrors the right except that a
port for liver retraction is not needed. After port placement, transperitoneal
LNU is performed in a standard manner and our detailed technique has been
published previously (5). For subsequent cystectomy and bilateral lymph
node dissection, the patient is re-positioned in a low lithotomy position
with a full Trendelenburg tilt. The entire surgical field is re-prepared
and re-draped for the lower urinary tract portion of the procedure. Laparoscopic
radical cystectomy with bilateral limited or extended lymph node dissection
is then performed as previously described (12,13). A 12 mm port site is
incised vertically in the midline above the umbilicus to be used as the
camera port (Port 6 depicted in Figure-1). Later, this port is extended
periumbilically for intact specimen removal and performance of all bowel
work including creation of the neobladder or ileal conduit as well as
re-establishment of bowel continuity.
RESULTS
A
total of 8 patients (7 males and 1 female) with a median age of 76.5 years
(range 65 to 79) underwent LNU and LRC with urinary diversion in the same
session. The indication for upper tract surgery was synchronous TCC in
6 patients (unilateral nephroureterectomy) and end-stage renal disease
in 2 patients (bilateral nephroureterectomy). Preoperatively, there was
a previous history of muscle-invasive or recurrent superficial TCC of
the bladder in all patients. Demographic data are detailed in Table-1.
Seven (87.5%) patients were classified as ASA score ≥ 3. Of the
patients, 7 (87.5%) underwent previous abdominal surgery.

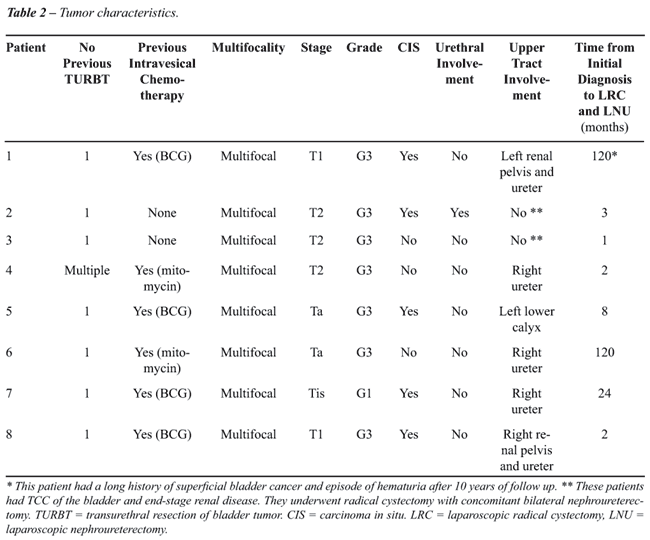
Preoperative tumor characteristics are presented
in Table-2. Six patients (75%) had a previous history of intravesical
chemo/immunotherapy (mitomycin-2, BCG-4). Five patients (62.5%) presented
with carcinoma in situ and 1 patient (12.5 %) had a positive urethral
biopsy for tumor. Site of upper tract tumor in 6 patients (right-4, left-2)
included ureter in 3, calyx in 1 and multiple locations in 2.
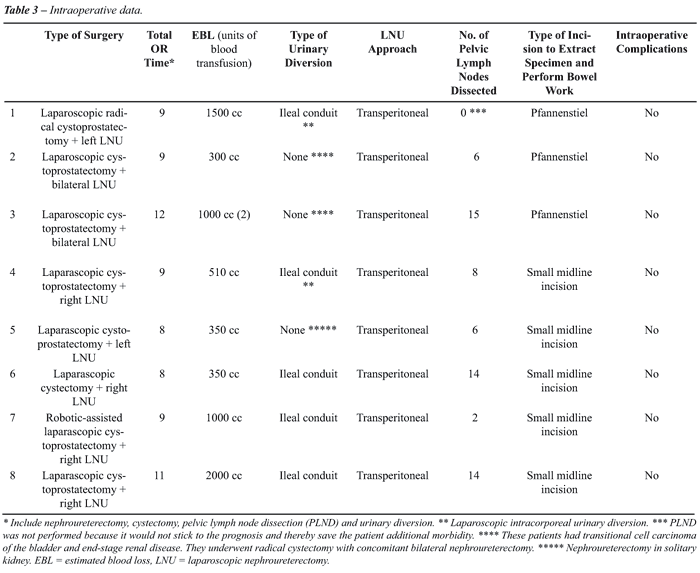
Median total operative time, which included
LNU, LRC, pelvic lymph node dissection and urinary diversion, was 9 hours
(range 8 to 12). Median estimated blood loss and hospital stay were 755
mL (range 300 to 2000) and 7.5 days (range 4 to 90), respectively. All
8 cases were technically successful without the need to open conversion.
There were no intraoperative complications. Table-3 summarizes the intraoperative
data.
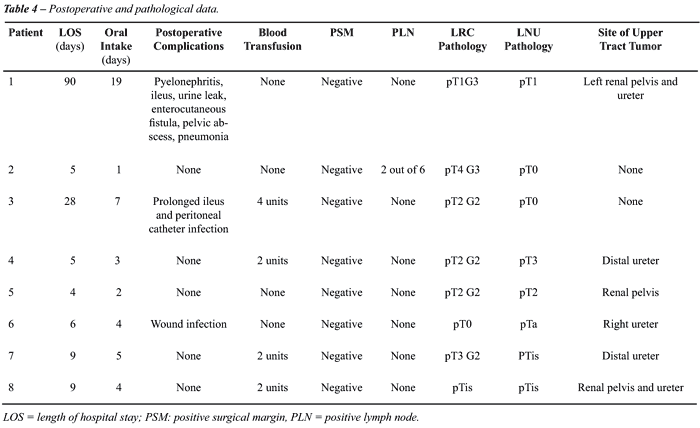
Postoperatively, 2 (25%) patients had minor
complications: prolonged ileus and peritoneal catheter infection in one
and wound infection in the other. There was one (12.5%) major complication:
sepsis due to peritonitis from an enterocutaneous fistula and pelvic abscess.
Median time for resumption to oral intake was 4 days (range 1 to 19).
Table-4 demonstrates the postoperative and pathological data.
Median follow-up was 9 months (range 1 to
45). Of the 6 patients undergoing unilateral LNU, 2 (33.4%) required dialysis
(one that had a previous contralateral nephrectomy and another due to
postoperative renal failure and sepsis). There were no cases of local
recurrence and only one (12.5%) patient developed distant metastasis and
died 8 months postoperatively. Four other patients have died during follow-up
including one during hospital stay, 2 from unknown cause (at 1 and 36
months) and 1 from cardiac disease at 45 months. The patient who died
during the hospital stay developed an enterocutaneous fistula due to a
small bowel perforation proximal to the ileal anastomosis at postoperative
day 7. He underwent fistula resection and drainage of an abscess. However,
the urine leak was consistent and he, therefore, underwent a right percutaneous
nephrostomy tube placement. However, this patient developed sepsis and
renal failure and died at 90 days after surgery. Other patient who died
at 1 month was discharged 28 days after surgery and died 2 days later
from unexplained causes. The family did not grant permission for a postmortem
evaluation. Three patients are alive with an overall survival and cancer
specific survival rate of 37.5% and 87.5%, respectively.
COMMENTS
Synchronous
or metachronous presentation of TCC in the upper and lower genitourinary
tract has been reported at varying rates throughout the literature. In
1989, Olbring et al. reported 11 cases (1.7%) of subsequent TCC of the
renal pelvis or ureter in 657 patients with bladder cancer (14). Of 1,529
patients with primary superficial bladder tumors, Rodriguez et al. reported
a 2.6% incidence of upper tract urothelial cancer (15). Herr et al. reviewed
a cohort of 86 patients with bladder tumor followed for at least 15 years
and found that 21% developed UUT tumors at a median of 7.3 years (16).
Accordingly, they have recommended lifelong upper tract surveillance for
urothelial cancer in patients with bladder tumor. Miyake et al. reported
an incidence of 13.2% of simultaneous bladder and UUT tumors in a total
of 106 cases (17). From our report, we noted a 7.9% (6 in 76 for our entire
LRC series to date) incidence of concurrent UUT TCC in our LRC series.
Simultaneous nephroureterectomy, radical
cystectomy and bilateral pelvic lymph node dissection is a challenging
surgical procedure independent of the approach. The patients are often
high-risk surgical candidates as demonstrated by the 87.5 % of patients
classified as ASA score ≥ 3 in our series. With regards the technique
used, special note must be made for the need to re-position the patient
between the nephroureterectomy and cystectomy portions. Moreover, the
ureter is never divided during the entire procedure and the urethra at
the prostate apex should be sewn shut for intact specimen extraction to
prevent any tumor spillage. In our series, 7 (87.5 %) patients underwent
previous abdominal surgery suggesting that previous surgery was not a
contraindication for the laparoscopic approach.
The various options for the urinary diversion
portion of the procedure depend on clinical condition of the patient,
the status of the urethra, patient preference and surgeon experience.
In this series, all patients primarily underwent extracorporeal ileal
conduit urinary diversion because they were all elderly and at high surgical
risk with multiple medical and surgical co-morbidities. Our recent report
confirms that the open-assisted laparoscopic approach for urinary diversion
portion of the procedure is technically more efficient and associated
with a quicker recovery profile and decreased complication rates compared
with the pure laparoscopic approach during LRC (18).
The extended pelvic lymph node dissection
during LRC, adhering to established oncological principles, has been previously
shown to be technically feasible (13). The survival appears to be better
in patients in whom > 14 lymph nodes were removed (19). In our series,
a limited lymphadenectomy was used in patients with a high-risk surgical
or in technically difficult cases (i.e. prior surgery) revealing a median
yield of 6 lymph nodes. Extended pelvic lymphadenectomy was used later
with our evolving experience in patients with better clinical conditions
with an increased median yield of 14 lymph nodes. Only one patient had
positive nodes. We stress our pelvic lymph node dissection was limited
in our early experience.
A simultaneous, bilateral approach is justified
in patients with ESRD, because synchronous upper tract TCC has been reported
to be more frequent in patients with renal insufficiency (20). In that
group, concomitant radical cystectomy with bilateral nephroureterectomy
avoids the need for urinary diversion and removes almost all urothelium
at risk for tumor recurrence. Care should be taken during renal dissection,
mainly in patients with previous surgery, to prevent injury to the adrenal
glands for subsequent adrenal insufficiency risk. The specimen can be
removed through a Pfannenstiel incision. In female patients, extraction
of the specimen en bloc through the vagina is a viable option (21).
For upper tract surgery, the conventional
advantages of the laparoscopic approach include earlier resumption of
oral intake, reduced narcotic analgesia requirement and decreased length
of hospital stay (22).
CONCLUSIONS
In this study, we have demonstrated the technical feasibility of simultaneous laparoscopic unilateral or bilateral nephroureterectomy and radical cystectomy and urinary diversion in patients with concomitant upper tract TCC or ESRD and bladder cancer. A greater number of patients and increased experience are needed to reduce the total operative duration and complications. Further studies are required to validate our results.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Landis SH, Murray T, Bolden S, Wingo PA: Cancer statistics, 1999. CA Cancer J Clin. 1999; 49: 8-31.
- Turna B, Aron M, Haber GP, Gill IS, Kaouk JH: Robotic radical cystectomy. Arch Esp Urol. 2007; 60: 439-48.
- Palou J, Rodríguez-Rubio F, Huguet J, Segarra J, Ribal MJ, Alcaraz A, et al.: Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. J Urol. 2005; 174: 859-61; discussion 861.
- Clayman RV, Kavoussi LR, Figenshau RS, Chandhoke PS, Albala DM: Laparoscopic nephroureterectomy: initial clinical case report. J Laparoendosc Surg. 1991; 1: 343-9.
- Gill IS, Sung GT, Hobart MG, Savage SJ, Meraney AM, Schweizer DK, et al.: Laparoscopic radical nephroureterectomy for upper tract transitional cell carcinoma: the Cleveland Clinic experience. J Urol. 2000; 164: 1513-22.
- Shalhav AL, Dunn MD, Portis AJ, Elbahnasy AM, McDougall EM, Clayman RV: Laparoscopic nephroureterectomy for upper tract transitional cell cancer: the Washington University experience. J Urol. 2000; 163: 1100-4.
- Rassweiler JJ, Schulze M, Marrero R, Frede T, Palou Redorta J, Bassi P: Laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: is it better than open surgery? Eur Urol. 2004; 46: 690-7.
- Manabe D, Saika T, Ebara S, Uehara S, Nagai A, Fujita R, et al.: Comparative study of oncologic outcome of laparoscopic nephroureterectomy and standard nephroureterectomy for upper urinary tract transitional cell carcinoma. Urology. 2007; 69: 457-61.
- Wu CF, Shee JJ, Ho DR, Chen WC, Chen CS: Different treatment strategies for end stage renal disease in patients with transitional cell carcinoma. J Urol. 2004; 171: 126-9.
- Takehara K, Nishikido M, Koga S, Miyata Y, Harada T, Tamaru N, et al.: Multifocal transitional cell carcinoma associated with renal cell carcinoma in a patient on long-term haemodialysis. Nephrol Dial Transplant. 2002; 17: 1692-4.
- Holton MR, Van Zijl PS, Oberle WT, Jacobs SC, Sklar GN: Complete urinary tract extirpation: the University of Maryland experience. Urology. 2006; 68: 65-9.
- Matin SF, Gill IS: Laparoscopic radical cystectomy with urinary diversion: completely intracorporeal technique. J Endourol. 2002; 16: 335-41; discussion 341.
- Finelli A, Gill IS, Desai MM, Moinzadeh A, Magi-Galluzzi C, Kaouk JH: Laparoscopic extended pelvic lymphadenectomy for bladder cancer: technique and initial outcomes. J Urol. 2004; 172: 1809-12.
- Oldbring J, Glifberg I, Mikulowski P, Hellsten S: Carcinoma of the renal pelvis and ureter following bladder carcinoma: frequency, risk factors and clinicopathological findings. J Urol. 1989; 141: 1311-3.
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, Huguet-Pérez J, Vicente-Rodríguez J: Upper urinary tract tumors after primary superficial bladder tumors: prognostic factors and risk groups. J Urol. 2000; 164: 1183-7.
- Herr HW, Cookson MS, Soloway SM: Upper tract tumors in patients with primary bladder cancer followed for 15 years. J Urol. 1996; 156: 1286-7.
- Miyake H, Hara I, Arakawa S, Kamidono S: A clinicopathological study of bladder cancer associated with upper urinary tract. BJU Int. 2000; 85: 37-41.
- Haber GP, Campbell SC, Colombo JR Jr, Fergany AF, Aron M, Kaouk J, et al.: Perioperative outcomes with laparoscopic radical cystectomy: “pure laparoscopic” and “open-assisted laparoscopic” approaches. Urology. 2007; 70: 910-5.
- Herr HW: Extent of surgery and pathology evaluation has an impact on bladder cancer outcomes after radical cystectomy. Urology. 2003; 61: 105-8.
- Chang CH, Yang CM, Yang AH: Renal diagnosis of chronic hemodialysis patients with urinary tract transitional cell carcinoma in Taiwan. Cancer. 2007; 109: 1487-92.
- Yuan LH, Chung HJ, Chen KK: Laparoscopic radical cystectomy combined with bilateral nephroureterectomy and specimen extraction through the vagina. J Chin Med Assoc. 2007; 70: 260-1.
- Portis AJ, Elnady M, Clayman RV: Laparoscopic radical/total nephrectomy: a decade of progress. J Endourol. 2001; 15: 345-54; discussion 375-6.
____________________
Accepted after revision:
February 12, 2008
_______________________
Correspondence address:
Dr. Rodrigo Frota
Section of Laparoscopic and Robotic Surgery
The Cleveland Clinic Foundation
9500 Euclid Avenue/A100
Cleveland, OH, 44195, USA
Fax: + 1 216 445-7031
E-mail:rodrigofrotaf@gmail.com
EDITORIAL COMMENT
Recently,
the laparoscopic approach has gained acceptance and more robust data support
for radical cystectomy with pelvic lymphadenectomy. The authors should
be commended for presenting the feasibility of simultaneous laparoscopic
nephroureterectomy and radical cystectomy in a very selective subset of
patients from a single, tertiary referral institution with high-volume
laparoscopic surgery for urologic malignancy. In this initial series,
we noted a high morbidity with two procedure related deaths (< 30 days
after discharge) probably due the advanced age and comorbidities of the
study subjects, combined with the surgical challenging scenario.
Additionally, this study covers a long timeframe
so the major complications observed in cases 1 and 3 might be related
to the learning curve of this complex procedure. From the technical standpoint,
we should emphasize the high rate of previous pelvic/abdominal surgery,
and that not all diversions were performed in an open-assisted manner
that is currently the standard-of-care for laparoscopic radical cystectomy,
fact that may be contributed for a longer operative time and postoperative
complication. Moreover, the extension of the lymphadenectomy was not ideal,
what can potentially compromise the specific-survival in these patients.
As the authors concluded, large studies are necessary before we can have
further conclusions on these preliminary results.
Dr. José Roberto Colombo and
Dr. Anuar Ibrahim Mitre
University of Sao Paulo, USP
Sao Paulo, Brazil
E-mail: anuar@mitre.com.br