ACTIVITY
OF ANTIOXIDANT ENZYMES IN SEMINAL PLASMA AND THEIR RELATIONSHIP WITH LIPID
PEROXIDATION OF SPERMATOZOA
(
Download pdf )
HEIDAR TAVILANI, MOHAMAD T. GOODARZI, ASAD VAISI-RAYGANI, SAEEDEH SALIMI, TAGHI HASSANZADEH
Department of Biochemistry (HT, MTG, THG), Medical School, Hamadan University of Medical Sciences, Hamadan, Iran, Department of Clinical Biochemistry (AVR), Faculty of Medicine, Kermanshah University of Medical Science, Kermanshah, Iran and Department of Biochemistry (SS), School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
ABSTRACT
Purpose:
To determine the activity of seminal plasma catalase (CAT), superoxide
dismutase (SOD) and glutathione peroxidase (GPX) and their relationship
with malondialdehyde (MDA), as a marker of lipid peroxidation, content
of spermatozoa and seminal plasma in normozoospermic and asthenozoospermic
males.
Materials and Methods: Semen samples were
obtained from 15 normozoospermic and 30 asthenozoospermic men.
Results: We observed inverse correlations
between activities of CAT (k/mL) and SOD (U/mL) in seminal plasma with
MDA content of spermatozoa from normozoospermic samples (r =- 0.43, p
< 0.05 and r =- 0.5, p < 0.05, respectively). Significant correlations
were observed between total activity CAT (k/total seminal plasma) with
total SOD (U/total seminal plasma) and GPX activity (mU/total seminal
plasma) in seminal plasma from normozoospermic samples (r = 0.67, p =
0.008 and r = 0.455, p = 0.047, respectively). Furthermore, we found positive
correlations between total activities of CAT, SOD and GPX with total content
of MDA in seminal plasma (nmoL/total seminal plasma) from normozoospermic
samples (r = 0.67, p = 0.003; r = 0.73, p = 0.003; r = 0.74, p = 0.004,
respectively). In asthenozoospermic samples, there were no significant
correlations observed between activities of CAT (k/mL), SOD (U/mL) and
GPX (mU/mL) of seminal plasma with MDA content of spermatozoa. However,
we found significant correlations between total activities of CAT (k/total
seminal plasma) and SOD (U/total seminal plasma) with total content of
MDA in seminal plasma (r = 0.4, p = 0.018 and r = 0.34, p = 0.03, respectively).
Conclusion: These findings indicate a protective
role for antioxidant enzymes of seminal plasma against lipid peroxidation
of spermatozoa in normozoospermic samples.
Key
words: catalase; superoxide dismutase; glutathione peroxidase;
semen; malondialdehyde
Int Braz J Urol. 2008; 34: 485-91
INTRODUCTION
Aerobic
metabolism of human sperm produces various reactive oxygen species (ROS),
which are potentially harmful to the sperm plasma membrane with its high
content of polyunsaturated fatty acids (1-3). There is growing evidence
that lipid peroxidation damage to the plasma membrane of spermatozoa plays
an important role in the mechanism of male infertility (4-6). The toxic
lipid peroxides are known to cause various impairments of the sperm cell,
such as membrane damage and decrease in motility (7,8). Control of lipid
peroxidation in the male reproductive tract is exerted by antioxidant
molecules and protective enzymes within the spermatozoa and seminal plasma
(9). Seminal plasma contains enzymatic ROS scavengers such as superoxide
dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT). These
enzymes act as an antioxidant and inhibitor of lipid peroxidation. Thus,
peroxidative damage in spermatozoa not only depends on ROS production,
but also on sperm and seminal plasma antioxidant defenses (10).
The question of whether seminal plasma SOD,
GPX and CAT can act coordinately to protect human spermatozoa from lipid
peroxidation has not to date been systematically addressed, although the
presence of SOD, GPX and CAT activity in seminal plasma from fertile and
infertile men has been reported (11-15). Since lipid peroxidation leads
to loss of motility in human spermatozoa, the possibility exists that
asthenozoospermic sperm suffers from the lack of protection against lipid
peroxidation due to lack of adequate or non-coordination between SOD,
GPX and CAT activity in seminal plasma. Whether the protective role of
seminal antioxidant enzymes, against peroxidation, can affect products
of spermatozoa or seminal plasma by lipid peroxidation remains unknown?
The main objective of this study was to determine the activity of seminal
plasma GPX, SOD and CAT and their relationship to MDA, as a marker of
lipid peroxidation, content of spermatozoa and seminal plasma in normozoospermic
and asthenozoospermic males.
MATERIALS AND METHODS
Semen Samples
Semen specimens were obtained in 30 asthenozoospermic patients who attended the Omid Fertility Clinic for infertility evaluation. In addition, 15 healthy men with normal semen parameters according to World Health Organization (WHO) criteria were enrolled as controls (16). The two groups were similar as regards mean age (20-40 years of age). Patients had no systemic diseases were non smokers and had no alcohol dependence, and none were taking an oral antioxidant supplement for three months prior tot the study. Patients fulfilling the inclusion criteria were asked to participate in this research project, which was duly explained to them. Written informed consent was obtained from all enrollees, according to the criteria of the Ethical Committee of Tehran University of Medical Sciences. All semen samples were collected by masturbation following 3 days of abstinence. After liquefaction, semen volume, sperm concentration (hemocytometer), total sperm count, morphology (Pap smear), motility grades: a (rapid progressive), b (slow progressive), c (non-progressive), d (immotile) were determined using WHO standard procedures (16). All major determinations were carried out in duplicate. Semen samples with more than 1×106/mL neutrophils using peroxidase staining (16) or other round cells were excluded. Asthenozoospermia was indicated by a sperm concentration of ≥ 20×106/mL and motility (grade a+b) of < 50%, irrespective of the morphology results. Normozoospermia was indicated by a sperm concentration of ≥ 20×106/mL and a motility (grade a+b) ≥ 50% and a normal morphology of ≥ 14%. Following semen analysis, a volume of semen containing at least 50 million sperm (or more) was transferred into a conical centrifuge tube and was centrifuged at 1000 × g for 10 min at room temperature. Immediately after the centrifugation, the supernatant was collected and stored at -80º C and the pellet from each sample was resuspended in 0.2 mL phosphate buffer saline (17).
Measurement of Antioxidant Enzymes Activity
Seminal
plasma SOD activity was measured using a Ransod kit (Randox Laboratories,
Crumlin, U.K.) with xanthine and xanthine oxidase to generate superoxide
radicals which react with 2- (4 - iodophenyl) - 3 - (4- nitrophenol)-5-phenyltetrazolium
chloride (I.N.T) to form a red formazan dye. Seminal plasma was diluted
31-fold with l0 mM phosphate buffer, pH 7. One unit of SOD was the amount
that caused a 50% inhibition in the rate of I.N.T. reduction. The SOD
activity was expressed as specific activity (U/mL seminal plasma) and
total activity (U/total seminal plasma).
Seminal plasma GPX was measured by a Ransel
kit (Randox Laboratories Ltd., London, U.K.). GPX catalyses the oxidation
of glutathione by cumene hydroperoxide. In the presence of glutathione
reductase and NADPH, the oxidized glutathione was immediately converted
to the reduced form with a concomitant oxidation of NADPH to NADP+.
The decrease in absorbance at 340 nm was measured. The GPX activity was
expressed as specific activity (mU/mL seminal plasma) and total activity
(mU/total seminal plasma).
Catalase activity was measured according
to Abei (18) by monitoring the initial rate of disappearance of hydrogen
peroxide (initial concentration 10 mM) at 240 nm. The catalase activity
was expressed as specific activity (k/mL seminal plasma) and total activity
(k/total seminal plasma).
Measurement of Malondialdehyde Levels
Lipid peroxidation in spermatozoa and seminal plasma was measured by reaction of thiobarbiuric acid (TBA) with malondialdehyde (MDA) according to Yagi (19). Content of MDA was measured spectrofluorometrically using a Jasco (FP-6200) spectrofluorometer (excitation 515 nm, emission 553 nm). The MDA fluorescence intensity of spermatozoa and seminal plasma was determined using various concentrations of tetraethoxypropane as standards. The results were expressed as nmoL MDA/10×106 cells, nmoL MDA/mL seminal plasma and nmoL MDA/total seminal plasma.
Statistical Analysis
Due to the fact that sperm concentration, motility, morphology, MDA and various other determined semen parameters were not normally distributed, the Mann-Whitney U test was applied to compare the asthenozoospermic and normozoospermic groups. To assess seminal plasma CAT, SOD, GPX activities and sperm count, one tailed two-independent sample t-test was used. Correlation between variables was assessed using non-parametric Spearman’s coefficient (r). Data were expressed M ± Standard Error.
RESULTS
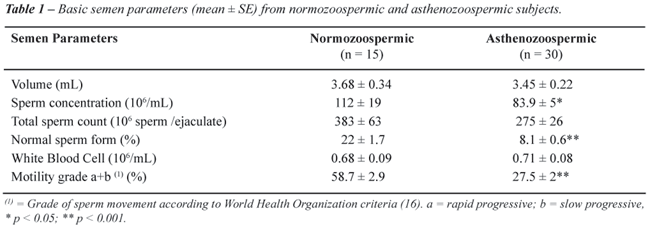
The
semen profiles of normozoospermic and asthenozoospermic samples are shown
in Table-1. Percent of motility grade a+b and spermatozoa with normal
morphology was higher in normozoospermic compared to asthenozoospermic
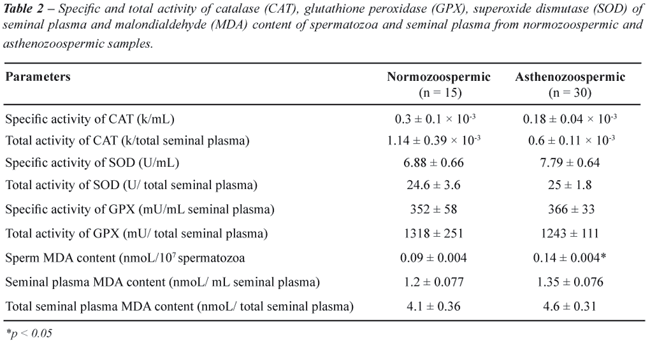
samples (p < 0.001). Results of seminal plasma CAT, SOD and GPX activities
in normozoospermic and asthenozoospermic groups are shown in Table-2.
Mean seminal plasma specific and total activity of SOD, GPX and CAT were
not significantly different in two groups. MDA content in the spermatozoa
of asthenozoospermic was significantly higher than in normozoospermic
samples (0.14 ± 0.004 and 0.09 ± 0.004 nmoL/107
spermatozoa, respectively). The mean ± SE value of MDA in the seminal
plasma of asthenozoospermic and normozoospermic were not significantly
different (Table-2).
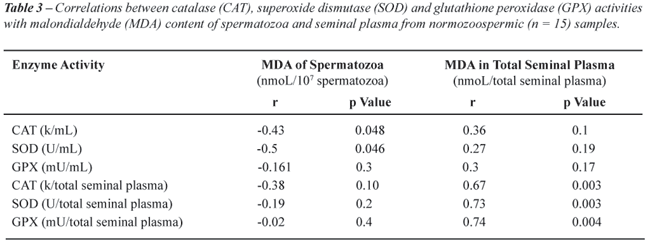
Correlations between CAT, SOD and GPX activities
with MDA content of spermatozoa and seminal plasma from normozoospermic
samples are shown in Table-3. There were negative and significant correlations
between activities of CAT and SOD in seminal plasma with MDA content of
spermatozoa from normozoospermic samples. In addition, we observed high
positive correlations between total activities of CAT, SOD and GPX with
total content of MDA from seminal plasma.
In asthenozoospermic samples, there were
no significant correlations between specific activities of CAT, SOD and
GPX of seminal plasma with MDA content of spermatozoa (Table-4). However,
we found positive and significant correlations between total activities
of CAT and SOD with total content of MDA in seminal plasma (Table-4).

Significant correlations were found between
total activity CAT with total activity SOD and total activity GPX in seminal
plasma from normozoospermic samples (r = 0.67, p = 0.008 and r = 0.455,
p < 0.05, respectively). In addition, there was a significant correlation
between specific activity CAT and specific activity SOD in normozoospermic
samples (r = 0.58, p = 0.022). Moreover, we observed a significant correlation
between total CAT and SOD activity in seminal plasma of asthenozoospermic
samples (r = 0.33, p < 0.05).
COMMENTS
In
the present study, we were able to determine the SOD, GPX and CAT activity
in the seminal plasma and the MDA content of the spermatozoa and seminal
plasma in normozoospermic and asthenozoospermic samples. Jones et al.
showed that the mechanism by which oxidative stress induced motility loss
in mammalian spermatozoa involved the induction of peroxidative damage
to the sperm plasma membrane (1). Human spermatozoa are particularly vulnerable
to lipid peroxidation because their plasma membranes are enriched with
polyunsaturated fatty acids, particularly docosahexaenoic acid with six
double bonds (6,20). These polyunsaturated fatty acids are essential to
produce plasma membrane fluidity that is required to participate in the
membrane fusion events associated with fertilization (1).
Our study showed that asthenozoospermic
men compared with normozoospermic do not have deficient seminal plasma
SOD, GPX and CAT activities. In contrast, there were no significant differences
between specific and total activity of SOD, GPX and CAT in seminal plasma
of two groups. Different studies have investigated antioxidant enzymes
of seminal plasma in asthenozoospermic samples or other altered semen
parameters but their results remain controversial (11-15). Results based
on this study showed a negative correlation between specific activity
of CAT and SOD with MDA content of spermatozoa from normozoospermic samples.
This observation suggests that CAT and SOD of seminal plasma may play
a role in the protection against lipid peroxidation in the normozoospermic
samples. In our study, we observed higher content of lipid peroxidation
product malondialdehyde (MDA) in spermatozoa of asthenozoospermic compared
with normozoospermic samples (p < 0.05). Although, the difference between
MDA of seminal plasma was not significant between two groups. In addition,
we did not find any significant correlation between spermatozoa MDA and
activity of antioxidant enzymes of seminal plasma. Moreover, the activity
of seminal antioxidant enzymes could not have protected spermatozoa from
asthenozoospermic samples against lipid peroxidation. Our results are
in agreement with Jones et al. who reported that the addition of SOD,
GPX and CAT to the medium of spermatozoa (which contain generating system
of oxygen free radicals; sodium ascorbate and FeSO4) did not inhibit MDA
formation (21). There is evidence for transferring of various proteins
to the spermatozoa, and the role of post testicular maturation of the
sperm cells have been well documented (22). We suggest that in the normozoospermic
samples, the membrane structure of spermatozoa is influenced to allow
adsorption of seminal plasma CAT and SOD onto the membrane, thereby providing
the protective action of CAT and SOD against lipid peroxidation. However,
in asthenozoospermic samples, seminal antioxidant enzymes cannot be adsorbed
to the plasma membrane of spermatozoa. Sperm membrane has been reported
to be adversely affected by peroxidation of polyunsaturated fatty acids
and accumulation of organic hydroperoides (21). Since, we found higher
content of lipid peroxidation product (MDA) in asthenozoospermic samples,
we suggest that membrane of spermatozoa was affected by lipid peroxidation
and thereby could not have adsorbed antioxidant enzymes of seminal plasma.
While there may be many reasons for increased lipid peroxidation product
in spermatozoa from asthenozoospermic males, one reason may be partly
due to non adsorption of seminal antioxidant enzymes to spermatozoa membrane
and subsequent reduction in lipid protection.
In this study, we found a positive correlation
between total activity of CAT, SOD and GPX with total content of MDA in
seminal plasma (nmoL/total seminal plasma) from normozoospermic samples.
In addition, our data showed the significant correlation between total
activity of CAT with total activity of SOD and GPX in normozoospermic
samples. These findings may indicate a cooperation and coordination between
function of antioxidant enzymes in normozoospermic samples. We suggest
that the activity of seminal antioxidant enzymes may be regulated by MDA
content of seminal plasma. Thus, further studies are needed to clarify
the role of MDA on activity of antioxidant enzymes of seminal plasma from
normozoospermic and asthenozoospermic samples.
In conclusion, these findings indicate a
protective role for antioxidant enzyme of seminal plasma against lipid
peroxidation of spermatozoa in normozoospermic samples. We suspect that
under pathological conditions (e.g. asthenozoospermia) the activity of
seminal antioxidant enzymes can not protect spermatozoa and may cause
an increase of lipid peroxidation from spermatozoa.
ACKNOWLEDGEMENTS
The authors are grateful to Professor Bayard T. Storey, University of Pennsylvania, for his review of this manuscript. Research was supported by Hamadan University of Medical Sciences.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Jones R, Mann T, Sherins R: Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979; 31: 531-7.
- Cocuzza M, Sikka SC, Athayde KS, Agarwal A: Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol. 2007; 33: 603-21.
- Lenzi A, Gandini L, Picardo M, Tramer F, Sandri G, Panfili E: Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): scavenger mechanisms and possible scavenger therapies. Front Biosci. 2000; 5: E1-E15.
- Aitken RJ, Buckingham D, West K, Wu FC, Zikopoulos K, Richardson DW: Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil. 1992; 94: 451-62.
- Zalata A, Hafez T, Comhaire F: Evaluation of the role of reactive oxygen species in male infertility. Hum Reprod. 1995; 10: 1444-51.
- Khosrowbeygi A, Zarghami N: Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot Essent Fatty Acids. 2007; 77: 117-21.
- Alvarez JG, Touchstone JC, Blasco L, Storey BT: Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987; 8: 338-48.
- Aitken RJ, Harkiss D, Buckingham D: Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil. 1993; 98: 257-65.
- Storey BT: Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997; 3: 203-13.
- Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J: Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl. 2004; 6: 59-65.
- Zini A, Garrels K, Phang D: Antioxidant activity in the semen of fertile and infertile men. Urology. 2000; 55: 922-6.
- Sanocka D, Miesel R, Jedrzejczak P, Kurpisz MK: Oxidative stress and male infertility. J Androl. 1996; 17: 449-54.
- Hsieh YY, Sun YL, Chang CC, Lee YS, Tsai HD, Lin CS: Superoxide dismutase activities of spermatozoa and seminal plasma are not correlated with male infertility. J Clin Lab Anal. 2002; 16: 127-31.
- Alkan I, Simsek F, Haklar G, Kervancioglu E, Ozveri H, Yalçin S, et al.: Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility: relationship to seminal plasma antioxidants. J Urol. 1997; 157: 140-3.
- Khosrowbeygi A, Zarghami N: Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol. 2007; 7: 6.
- World Health Organization WHO: Laboratory Manual for the Examination of Human Semen and Semen - Cervical Mucus Interaction, 4 th (ed.), Cambridge University Press. 1999; p. 4-23.
- Engel S, Schreiner T, Petzoldt R: Lipid peroxidation in human spermatozoa and maintenance of progressive sperm motility. Andrologia. 1999; 31: 17-22.
- Aebi H: Catalase in vitro. Methods Enzymol. 1984; 105: 121-6.
- Yagi K: Assay for blood plasma or serum. Methods Enzymol. 1984; 105: 328-31.
- Tavilani H, Doosti M, Nourmohammadi I, Mahjub H, Vaisiraygani A, Salimi S, et al.: Lipid composition of spermatozoa in normozoospermic and asthenozoospermic males. Prostaglandins Leukot Essent Fatty Acids. 2007; 77: 45-50.
- Christova Y, James PS, Jones R: Lipid diffusion in sperm plasma membranes exposed to peroxidative injury from oxygen free radicals. Mol Reprod Dev. 2004; 68: 365-72.
- Saez F, Frenette G, Sullivan R: Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl. 2003; 24: 149-54.
____________________
Accepted after revision:
April 4, 2008
_______________________
Correspondence address:
Dr. Heidar Tavilani
Department of Biochemistry
Medical School, Hamadan Univ of Medical Sciences
Hamadan, Iran
Fax: + 98 811 827-6299
E-mail: tavilani@gmail.com