IS
CONTINENT URINARY DIVERSION FEASIBLE IN CHILDREN UNDER FIVE YEARS OF AGE?
(
Download pdf )
LUIZ L. BARBOSA, RIBERTO LIGUORI, SERGIO L. OTTONI, UBIRAJARA BARROSO JR, VALDEMAR ORTIZ, ANTONIO MACEDO JUNIOR
Division of Urology, Federal University of Sao Paulo, Sao Paulo, Brazil
ABSTRACT
Purpose:
To review our clinical experience with urinary continent catheterizable
reservoir in children under five years of age.
Materials and Methods: A total of 23 patients
(16 males, 7 females) with a median age of 3.64 years were evaluated.
Among these, 6 (26.08%) had a posterior urethral valve, 9 (39.13%) myelomeningocele,
4 (17.39%) bladder exstrophy, 2 (8.69%) genitourinary rabdomyosarcoma,
1 (4.34%) had spinal tumor and 1 (4.34%) an ano-rectal anomaly.
Results: Perioperative complications were
observed in four patients consisting of one febrile urinary tract infection,
one partial operative wound dehiscence, one partial stomal dehiscence
and one vesico-cutaneous fistula after a secondary exstrophy repair. The
overall long-term complications rate was 40.90% and consisted of two stomal
stenoses (9.09%), one neobladder mucosal extrusion (4.54%), three neobladder
calculi (13.63%) and persistence of urinary incontinence in three patients
(13.63%). The overall surgical revision was 36.36% and final continence
rate was 95.45% with mean follow-up of 39.95 months
Conclusion: Continent urinary diversion
is technically feasible even in small children, with acceptable rates
of complications.
Key
words: children; congenital anomalies; urinary diversion; continence
Int Braz J Urol. 2009; 35: 459-66
INTRODUCTION
The ability to void spontaneously
may be compromised in children with various congenital urologic abnormalities
(neurogenic bladder, posterior urethral valve and bladder exstrophy).
Although clean intermittent catheterization (CIC), pharmacological treatment
and overnight catheter drainage has changed the natural history of most
of these uropathies., it is not always possible to avoid renal deterioration
in these patients owing to a combination of high detrusor pressure, infection
and vesicoureteral reflux, moreover, urethral catheterization can be painful
or not possible in female patients bound to a wheel chair.
The use of continent urinary reservoirs
has gained wide acceptance, because when this procedure is combined with
CIC, patients are often able to achieve continence with low intravesical
pressure. If necessary, a continent catheterizable urinary reservoir and
a concomitant bladder neck procedure can be helpful to restore continence.
On the other hand, each type of augmentation
cystoplasty involves short-term and long-term morbidity (bacteriuria,
pyelonephritis, mucus production, stone disease, metabolic disturbances,
bladder rupture/perforation and malignancy) that may lead to impaired
linear growth in young children (1,2). To our knowledge, there is no consensus
in the literature as regards patients’ initial age when this procedure
can be safely performed. The majority of the series report bladder augmentation
in patients above 5 years of age (> 5 years).
We retrospectively present our experience
with orthotopic continent urinary diversion in children under five years
of age. We evaluated all continent catheterizable procedures that were
performed in our institution, to investigate if continent urinary diversion
is technically feasible and safe even in younger children.
MATERIALS AND
METHODS
The
records of children who underwent a Macedo’s continent catheterizable
reservoir below five years of age were retrospectively reviewed. A total
of 23 patients (16 males, 7 females) met the inclusion criteria in our
present series of 115 procedures. Patient age at surgery varied from 1
to 5 years with median age of 3.64 years. All children had previously
undergone urodynamic evaluation, except those with rabdomyosarcoma and
bladder exstrophy.
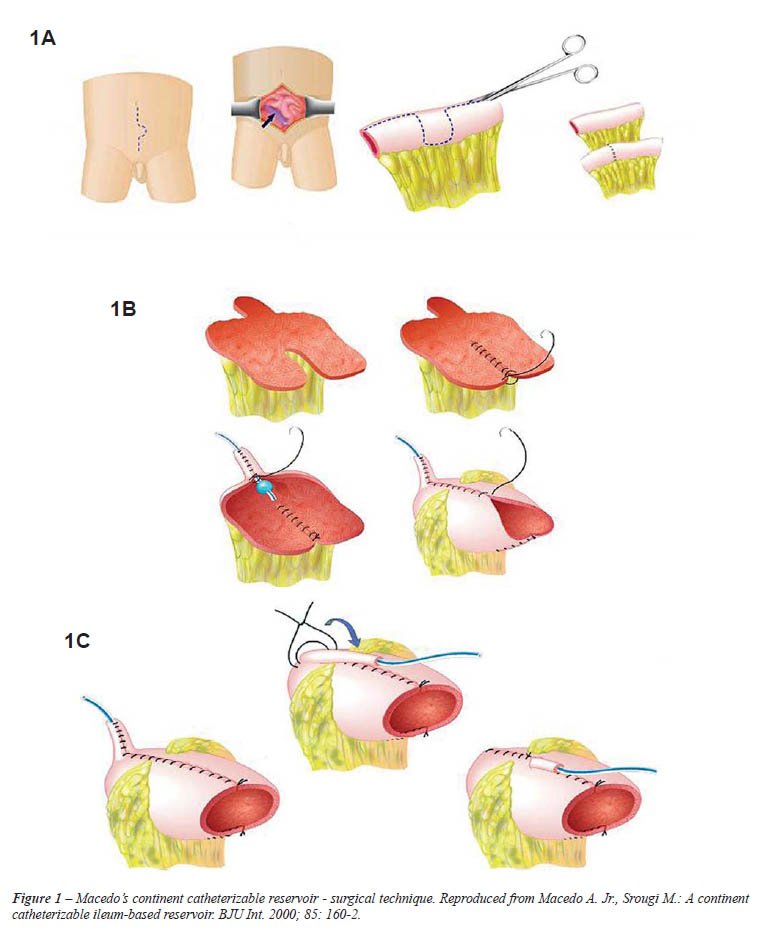
The technique consists of a continent catheterizable
ileum-based reservoir (3). A 30 cm segment of the distal ileum is isolated
and bowel continuity restored by end-to-end anastomosis. The detubularization
of the ileal segment follows the antimesenteric border of the intestine
up to the middle of the segment. Here the incision line continues transversally
to the anterior surface of the ileum, reaching the mesenteric border.
A 3 cm horizontal incision along the mesenteric side is continued before
returning to its usual direction at the antimesenteric border. The remainder
of the ileum is then opened longitudinally (Figure-1A). The 3 cm flap
from the anterior surface of the middle part of the ileum is mobilized
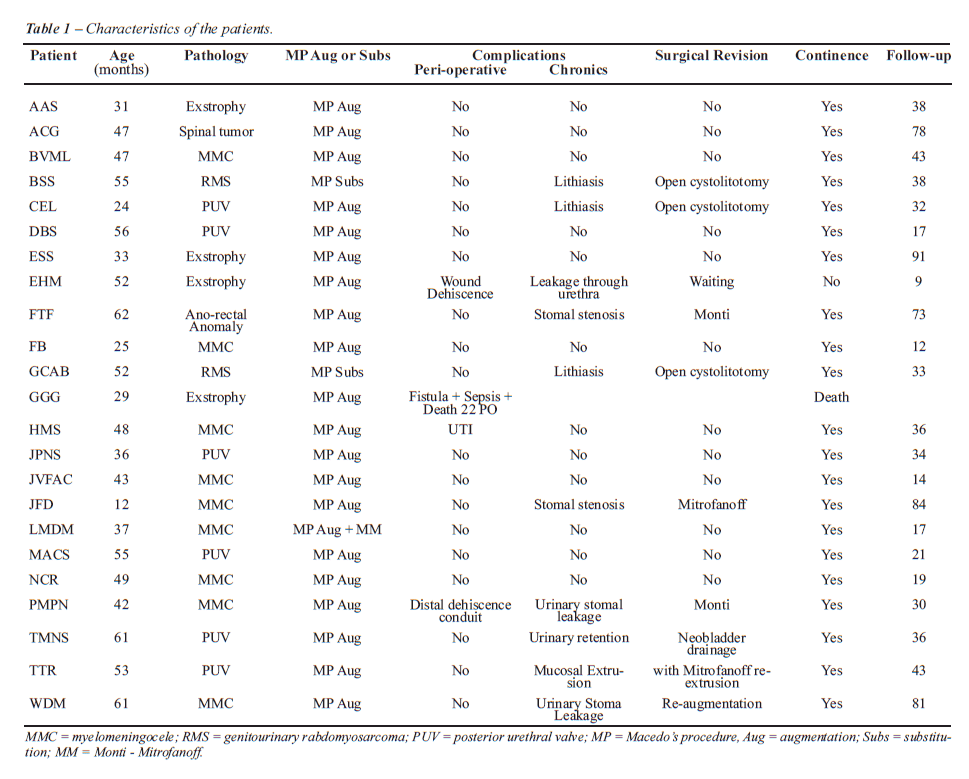
cranially, and tubularized around a 12F silicone Foley catheter (Figure-1B).
The continence valve mechanism is produced by embedding the tube over
a serous-lined extramural tunnel created by polypropylene 3/0 sutures
(Figure-1C). The distal end of the tube is anastomosed into a V-shaped
to the skin flap to avoid stomal stenosis. The reservoir is anastomosed
to the bladder or used as a substitute.
Surgical indications included patients with low bladder compliance and
capacity, filling detrusor pressure above 40 cm H2O, and/or urinary incontinence
that had failed to conservative management (CIC and anticolinergics).
Among the patients, 6 (26.08%) had posterior
urethral valve (PUV), 9 (39.13%) myelomeningocele, 4 (17.39%) bladder
exstrophy, 2 (8.69%) genitourinary rabdomyosarcoma, 1 (4.34%) spinal tumor
and 1(4.34%) had an ano-rectal anomaly (Table-1). Four patients (17.39%)
had undergone either a vesicostomy or nephrostomy prior to bladder augmentation.
One patient had bladder augmentation 14 months before kidney transplantation
(Table-1).
The procedure was performed with the ileum
in 21 patients (91.30%) and transverse colon in 2 patients (8.69%). The
colon was used in two cases of genitourinary rhabdomyosarcoma, due to
the risk of using a previous irradiated ileum to perform bladder substitution
(4). In only these two patients, the technique was used as a pouch whereas
in the others it was used as a bladder augmentation.
In one patient (4.34%), an antegrade left
colon enema procedure was performed in combination with bladder augmentation
due to fecal incontinence (5).
Two of the 21 patients (8.69%) underwent
a bladder neck procedure, due to persistent leakage and low detrusor leak
point pressure (6).
The patients’ medical records were
reviewed in order to define complication rates (acute and chronic), surgical
revision rate and final continence.
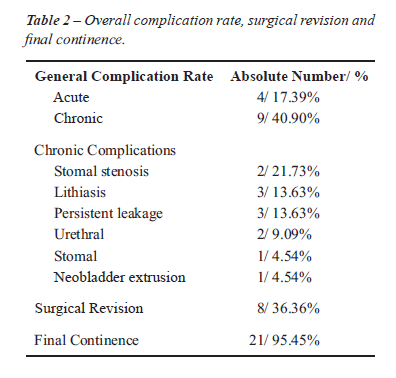
RESULTS
A perioperative complication
was observed in four patients consisting of one febrile urinary tract
infection, one partial operative wound dehiscence, one partial stomal
dehiscence and one vesico-cutaneous fistula after a secondary exstrophy
repair. This last patient, developed sepsis due to fungal infection and
did not survive.
The overall long-term complications rate
was 40.90% and consisted of two stomal stenoses (9.09%), one neobladder
mucosal extrusion (4.54%), three neobladder calculi (13.63%) and persistence
of urinary incontinence in three cases (13.63%), Table-2.
In the two cases of stomal stenosis, surgical revision was necessary in
both cases because progressive stomal dilatation failed.
The patient with neobladder mucosal extrusion
(4.54%) underwent redo closure and apendicovesicostomy.
Three patients presented with persistence
of urinary leakage (13.63%), two leaking through the stoma and one patient
through the urethra after failed bladder neck surgery. The patients with
stomal urinary leakage (9.09%) were reoperated: one patient underwent
a Monti procedure and the other a re-augmentation and they are currently
continent.
Three patients developed reservoir calculi
(13.63%), and underwent open cystolitotomy after failed endoscopic attempt.
All cases were found to have the calculi grown on a suture line presumably
associated to one of the seromuscular Prolene sutures of the tube valvular
mechanism.
One patient presented with late urinary
retention due to overdistention of the reservoir and tube outlet, and
a cystoscopy was necessary to help catheter passage through the stoma.
The overall surgical revision was 36.36%
and final continence rate was 95.45% with mean follow-up of 39.95 (9 to
91) months. The only incontinent patient was a complex exstrophy form
that failed a bladder neck procedure and is currently awaiting surgery
(Table-1).
COMMENTS
Successful continent abdominal
diversion requires a low pressure reservoir, a continent efferent channel
and a good cosmetic and non stenotic skin stoma.
The choice of the intestinal segment to
construct the reservoir is made according to surgeons’ experience,
but factors like incidence of electrolyte reabsorption and loss (acidosis
and alcalosis) certainly play a role in this procedure.
The creation of a continent catheterizable
conduit was initially described by Mitrofanoff, using the appendix as
a catheterizable stoma. Although the appendix is the most popular channel,
numerous other options have been reported (ureter, fallopian tubes and
tubularized colonic or bladder flaps) (7). In cases when the appendix
is absent, too short or has evidence of luminal stenosis, the search for
an alternative is imperative. The Yang-Monti procedure consists of using
a small segment of bowel (usually ileum) to create an efferent tube. A
2.0-2.5 cm segment of bowel is detubularized in the antimesenteric border
resulting in a tube of 6-7 cm in length, when transversely retubularized.
To date, the Yang-Monti technique is considered the substitute of choice
of the appendix for the Mitrofanoff principle (8).
In Mitrofanoff and Yang-Monti procedures,
when concomitant bladder augmentation or substitution is needed, two segments
of bowel must be prepared for creation of a neobladder and an efferent
tube. After that, both structures have to be joined together. The Macedo’s
continent catheterizable reservoir allows both procedures using the same
segment of the bowel. We recently performed an experimental study to define
the efficacy of the technique, in addition to other factors that may influence
continence of catheterizable channels using the principle of embedding
the outlet tube. From 20 pigs, colon specimens with 25 cm length were
obtained and a transverse flap in the average point of the intestine was
incised to create an efferent tube. A pressure study of both intra-luminal
surface and channel was then conducted during the filling, of the submersed
piece with environmental air in a water container, to define the efferent
channel continence. This study showed that angulation of channel with
colon, maintained by only one stitch was more important than a larger
extension of the valve, represented by 3 sutures in order to allow continence
to the efferent channel. This experimental study reproduced the clinical
situation in our patients and outlined the need of anchoring the reservoir
to the abdominal wall to retain the angulation of the stoma (9).
Leslie et al. (8) recently reviewed their
series of Monti urinary channels performed from 1997 to 2004. These authors
identified 168 patients with age of 10 months to 31 years at surgery.
Mean follow-up was 2, 6 years. In 168 patients, the ileum was used to
create the channel in 165 (98.2%), while sigmoid colon was used in the
other 3. A single Monti channel was created in 99 (58.9%) and a spiral
Monti in 66 (39.3%). A total of 37 open revisions were required (18.5%).Of
these 31 patients, 26 required only 1 open revision, 4 required 2 revisions,
and 1 patient required 3 revisions. Of the 152 patients, 148 were completely
continent (148 of 152 or 97, 4%), 1 had rare leakage, 2 had occasional
or intermittent leakage, and 1 continues to have frank leakage.
Thomas et al. (7) retrospectively reviewed
the continent catheterizable channel outcomes in their patients between
1998 and 2003. A total of 68 Mitrofanoff procedures were performed, using
appendix in 43% of cases, an ileal Yang-Monti tube in 38% and continent
cutaneous vesicostomy in 19%. Stomal stenosis occurred in 9 patients (13%)
within 1 to 24 months after surgery. False passages with catheterization
occurred in four patients (6%) with a mean follow-up of 6, 5 months. Of
the two patients with stomal stenosis both required surgical revision.
The final overall continence rate was 95.45%,
therefore similar to other series. Stomal leakage was found in two patients
(9.09%) requiring a surgical revision: redo procedure in one patient and
outlet channel revision (Monti tube) in the other. Both patients are presently
continent. The other case of urethral incontinence was an exstrophy patient
who is currently awaiting a second bladder neck procedure.
Persistent leakage and stomal complications,
mainly stenosis, are the most commonly reported pitfalls of continent
urinary diversion. Previous studies have examined the relationship between
stomal complications and type of conduit, type of reservoir, and type
and site of stoma. Stomal stenosis appears to be independent from all
of these variables (10).
It is well known that bladder augmentation
and catheterizable urinary diversion lead to an increased risk of urolithiasis.
Reasons for this include urinary stasis, mucus production, and chronic
bacteriuria. Our incidence of lithiasis was 13.63%, lower than other series.
At our institution, all parents are instructed to promote aggressive irrigation
of their children’s reservoir at least once every 24 hours with
100 to 150 mL of water. A nurse specialized in pediatric urology and stomal
care, monitors our patients regularly to make sure that parents follow
established guidelines.
Metcalfe et al. (11) published a series
of 500 bladder augmentations in children, revealing bladder stones formed
in 20 patients, 1% with a continent catheterizable channel compared to
10, and 3% without a channel. The increased risk of bladder calculi associated
with continent channels is in agreement with the study of Brough et al.
(12) which is probably due to incomplete emptying of the reservoir based
on nondependent catheterization. No difference in prevalence of stone
formation as regards ileal or sigmoid segments was reported.
Malignancy and enterocystoplasty is also
a growing concern and reports from pediatric bladder augmentation are
increasing in frequency. It is believed that the incidence of malignancy
will increase with longer follow-up. Pathogenesis likely involves nitrosamines
produced by bacteria in urine (13-15). Until the pathogenesis and the
etiology are better understood, the mainstay of prevention is regular
endoscopic surveillance. In the hope of preventing this disturbing event,
some authors have become more aggressive in managing recurrent urinary
tract infections and advocate annual surveillance cystoscopy to begin
at least 8 to 10 years after augmentation. The role of screening urinary
cytology has not been explored but it has merit and warrants further study
(11).
We recognize that our mean follow-up of
40 months is not long enough to speculate on long-term complications like
malignancy or nutritional deficits but this was not the scope of this
article. On the other hand, we believe that once a precise indication
for augmentation is established, it would make no difference in operating
the child after 5 years of within 3.6 years (mean age at present study).
It is important to stress that we are not advocating earlier surgery than
necessary but only reporting on clinical data retrospectively. We admit,
however, that this issue is controversial and many authors would argue
that they would leave patients with incontinent stomas or ureterostomies
until later on. We respect this argument but we regularly offer patients
both options and they in fact help us to decide which procedure to follow.
A second potential criticism on this paper
would be that early augmentation may increase work for the family simply
to achieve a goal (urinary continence) that other children of their age
have not yet reached. However, most of our data comprises patients with
myelomeningocele, posterior urethral valves and bladder exstrophy. As
we are aware, most of these patients are already undergoing CIC for preventing
upper urinary tract deterioration, so that excluding our 4 patients with
exstrophy, bladder reconstruction would not add much work in terms of
care, quite the contrary if we think that a abdominal stoma is much easier
to handle than performing urethral catheterization in wheel-chair bounded
children or boys with urethral sensitivity.
We would like to make clear that the scope
of this retrospective study was to look back in our bladder augmentation
database and evaluate if the rate of complications was higher in this
period of life. We are not advocating early surgery but this series comprises
a very complex population and most PUV and myelomeningocele patients had
upper urinary tract deterioration and recurrent urinary tract infection
and required treatment.
CONCLUSION
Our experience shows that continent catheterizable urinary diversion performed by Macedo’s technique is a safe procedure even in small patients below five years, guaranteeing renal function preservation, improvement in quality of life with an acceptable rate of complications. It is important that these patients and their parents have to be included in a nurse-assisted program of clean intermittent catheterization, and be stimulated to promote an adequate emptying of their reservoirs, diminishing the risks of bacteriuria, mucus production, lithiasis and stoma stenosis. Long-term results for children operated on under the age of 5 are still lacking but we do not expect much difference compared to that which is currently reported in the literature.
CONFLICT OF
INTEREST
None declared.
REFERENCES
- Gilbert SM, Hensle TW: Metabolic consequences and long-term complications of enterocystoplasty in children: a review. J Urol. 2005; 173: 1080-6.
- Feng AH, Kaar S, Elder JS: Influence of enterocystoplasty on linear growth in children with exstrophy. J Urol. 2002; 167: 2552-5; discussion 2555.
- Macedo A Jr, Srougi M: A continent catheterizable ileum-based reservoir. BJU Int. 2000; 85: 160-2.
- Freitas RG, Nobre YT, Macedo A Jr, Demarchi GT, Ortiz V, Srougi M: Continent urinary reconstruction in rhabdomyosarcoma: a new approach. J Pediatr Surg. 2004; 39: 1333-7.
- Calado AA, Macedo A Jr, Barroso U Jr, Netto JM, Liguori R, Hachul M, et al.: The Macedo-Malone antegrade continence enema procedure: early experience. J Urol. 2005; 173: 1340-4.
- Macedo A Jr, Garrone G, Leslie B, Liguori R, Ottoni SL, Ortiz V: A new method to augment the bladder, provide a catheterizable stoma and reconstruct the bladder neck in one operation from a single intestinal segment: The technique step-by-step. J Ped Urol 2006; 2: 84. Abstract no 17.
- Thomas JC, Dietrich MS, Trusler L, DeMarco RT, Pope JC 4th, Brock JW 3rd, et al.: Continent catheterizable channels and the timing of their complications. J Urol. 2006; 176: 1816-20; discussion 1820.
- Leslie JA, Dussinger AM, Meldrum KK: Creation of continence mechanisms (Mitrofanoff) without appendix: the Monti and spiral Monti procedures. Urol Oncol. 2007; 25: 148-53.
- Vilela ML, Furtado GS, Koh I, Poli-Figueiredo LF, Ortiz V, Srougi M, et al.: What is important for continent catheterizable stomas: angulations or extension? Int Braz J Urol. 2007; 33: 254-61; discussion 261-3.
- Rapoport D, Secord S, MacNeily AE: The challenge of pediatric continent urinary diversion. J Pediatr Surg. 2006; 41: 1113-7.
- Metcalfe PD, Cain MP, Kaefer M, Gilley DA, Meldrum KK, Misseri R, et al.: What is the need for additional bladder surgery after bladder augmentation in childhood? J Urol. 2006; 176: 1801-5; discussion 1805.
- Brough RJ, O’Flynn KJ, Fishwick J, Gough DC: Bladder washout and stone formation in paediatric enterocystoplasty. Eur Urol. 1998; 33: 500-2.
- Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC: Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004; 172: 1649-51; discussion 1651-2.
- Lane T, Shah J: Carcinoma following augmentation ileocystoplasty. Urol Int. 2000; 64: 31-2.
- Filmer RB, Spencer JR: Malignancies in bladder augmentations and intestinal conduits. J Urol. 1990; 143 :671-8.
____________________
Accepted
after revision:
March
10, 2009
_______________________
Correspondence
address:
Dr. Antonio
Macedo Jr.
Rua Maestro Cardim, 560 / 215
São Paulo, SP, 01323-000, Brazil
Fax: +55 11 3287-3954
E-mail: macedo.dcir@epm.br
EDITORIAL COMMENT
This retrospective series of children under age 5 years undergoing continent urinary diversion provides food for thought to readers. The short and long term complication rates, including stone formation, ongoing leakage, stomal stenosis and the need for further surgery are similar to those previously published from other centers regarding older patients. The surgical technique described is another novel variation of the Mitrofanoff principle. Clearly, the technical feasibility of performing this surgery in young patients has been established by the authors, but as they rightly point out, the necessity for such surgery at a young age remains debatable. The one death in their series, although perhaps a statistical aberration, speaks to the gravity of performing these procedures in young patients with often compromised reserves. Caution should be advised before considering continent urinary diversion as the standard approach to the young incontinent patient. These patients and their families are often well served with incontinent stomas, and pull-ups or pads until size, maturity and socialization mandate a surgical solution.
Dr.
Andrew E. MacNeily
BC Children’s Hospital
Vancouver, British Columbia
Canada
E-mail: amacneily@cw.bc.ca