MULTIMODAL
THERAPY FOR PAINFUL BLADDER SYNDROME / INTERSTITIAL CYSTITIS: PILOT STUDY
COMBINING BEHAVIORAL, PHARMACOLOGIC, AND ENDOSCOPIC THERAPIES
(
Download pdf )
ROBERT S. HANLEY, JOHN T. STOFFEL, RALPH M. ZAGHA, ARTHUR MOURTZINOS, JOHN F. BRESETTE
Anne Arundel Urology (RSH), Annapolis, Maryland, Department of Urology (JTS, AM, JFB), Lahey Clinic Medical Center, Burlington, Massachusetts, Department of Urology (RMZ), Florida Medical Center, Lauderdale Lakes, Florida, USA
ABSTRACT
Purpose: We evaluated the effectiveness of combining behavioral therapy,
pharmacologic therapy and endoscopic hydrodistension for treating painful
bladder syndrome / interstitial cystitis (PBS/IC).
Materials and Methods: Twenty-five patients
with PBS/IC were prospectively enrolled in a pilot multimodal behavioral,
pharmacologic and endoscopic treatment protocol. Behavioral modification
included diet recommendations, fluid restriction to 64 oz. /day, progressive
timed voiding and Kegel exercises. Oral pharmacologic therapy consisted
of daily doses of macrodantin 100 mg, hydroxyzine 10-20 mg and urised
4 tablets. Patients underwent endoscopic bladder hydrodistention under
anesthesia at least 2 weeks after protocol enrollment. Behavioral and
pharmacological treatments were continued after the hydrodistention. O’Leary-Sant
questionnaire scores were recorded before starting the protocol, after
pharmacologic/behavioral therapy, 2 months post-hydrodistension, and at
scheduled follow-up.
Results: Eighteen patients (72%) completed
the pilot multimodal treatment protocol and were followed for a mean of
10.2 months. All patients were female with a median age of 36.3 years
and had mean bladder capacity under anesthesia of 836 milliliters. Mean
O’Leary-Sant symptom index scores for baseline symptoms, after behavioral/pharmacologic
treatment, post-hydrodistension and during follow up were 12.5, 8.6, 7.0,
and 6.7 (p < 0.05). Mean O’Leary-Sant problem index scores for
baseline, after behavioral/pharmacologic treatment, post-hydrodistention
and during follow up were 12.7, 8.9, 6.7, and 7.7 (p < 0.05).
Conclusion: Our pilot multimodal protocol
of behavioral modification, pharmacologic therapy and endoscopic hydrodistention
demonstrated a significant progressive improvement in PBS/IC quality of
life scores, compared to a pre-treatment baseline. These results should
be validated in a larger, placebo controlled trial.
Key
words: cystitis, interstitial; pilot project; behavioral medicine;
endoscopy; pharmacology
Int Braz J Urol. 2009; 35: 467-74
INTRODUCTION
Painful
bladder syndrome / interstitial cystitis (PBS/IC) is defined by the International
Continence Society as “suprapubic pain related to bladder filling,
accompanied by other symptoms such as increased day- and nighttime frequency,
in the absence of proven urinary infection or other obvious pathology.
Interstitial cystitis is a PBS characterized by cystoscopic and morphological
findings not further defined.” (1). The pathophysiology of PBS/IC
remains unclear and investigators have attributed the severe symptoms
to a history of chronic urinary tract infections, leaky glycosaminoglycan
layers in the bladder, autoimmune inflammation, and/or neurogenic inflammation
(2). Because of the uncertainty surrounding the underlying PBS/IC pathophysiology,
there are multiple treatment options for patients with severe symptoms.
In general, PBS/IC treatments have focused on behavioral modifications,
pharmacotherapy, or endoscopic treatments. However, the efficacy of each
type of treatment is highly variable when examined as monotherapy (3).
In fact, PBS/IC may represent several different
etiologies presenting with common symptomatic endpoints. Consequently,
multimodal, treatment for PBS/IC may improve overall efficacy because
different pathophysiologies may require different treatment modalities.
Currently, there is little information in the literature on a multimodal
approach for treating PBS/IC. We have developed a pilot treatment program
that offers a simple combination of common, easy to implement behavioral
modification, pharmacologic, and endoscopic therapies for PBS/IC. The
goal of this pilot study was to determine if this combination of multimodality
therapy offered consistent, measurable relief for female patients presenting
with previously untreated symptomatic PBS/IC.
MATERIALS AND METHODS
Female
patients referred to the urogynecology clinics of three physicians for
irritative or painful bladder symptoms were screened for PBS/IC using
the ICS PBS/IC definition. The IC Database Study requirements were also
utilized for exclusion criteria to rule out confounding pathologies (4),
Table-1. Prior to pilot study enrollment, all patients underwent a complete
history and physical examination to rule out other potential sources for
bladder symptoms and all patients submitted urine specimens for culture
and cytology. Only female patients with a new diagnosis of PBS/IC and
the absence of other urologic pathologies were included in the study.
Patients unable to complete questionnaires or unwilling to agree to a
scheduled treatment plan were excluded from the study. The study was approved
by the institutional IRB.
The multimodal therapy used in this study
consisted of three established PBS/IC treatments: behavioral modification,
pharmacologic therapy, and endoscopic hydrodistension. Behavioral modification
and pharmacologic therapy were started at the time of patient enrollment.
Endoscopic therapy was initiated a minimum of 2 weeks afterwards.

Behavioral Modification
PBS/IC patients enrolled in the study were given 30 minutes of specific verbal instruction by an urogynecology physician or nurse using a set script. Patients were instructed to perform progressive timed voiding on a 2 to 3 hour schedule and instructed to limit liquid intake to 64 oz. per day, divided into 16 oz. per meal and 8 oz. between meals. For patients unable to hold urine for this interval, instructions were given to progressively increase urine storage time between voids by 15 minutes per week until the goal of a 2 to 3 hour interval was reached. All patients were also instructed to perform 15 pelvic muscle Kegal contractions twice a day. At the conclusion of the teaching session, all patients were given written instructions that summarized these behavioral regimens. An exclusionary list of foods that may exacerbate PBS/IC symptoms was discussed (5). If a patient identified a specific food or foods from the list that they believed may increase her bladder symptoms, recommendations were given to avoid this substance. At each successive clinic visit, an urogynecology nurse questioned the patient regarding compliance to each element of the behavioral therapy regimen and deviation from recommended therapy was noted.
Pharmacologic Therapy
At the first visit, all patients were also instructed on a specific oral pharmacologic regimen that included macrodantin 100 mg daily, hydroxyzine 10 to 20 mg daily, and Urised (methenamine, methylene blue, phenyl salicylate, benzoic acid, atropine sulfate, hyoscyamine) 4 tablets daily. Patients also continued pentosan polysulfate sodium 100 mg three times per day if their primary care physician had started this medication 6 months prior to the first urogynecology visit. Medication compliance was assessed by a urogynecology nurse at each clinical follow-up. Medication side effects and deviation from recommended therapy was recorded. No patients were prescribed additional narcotics/analgesics during the study.
Endoscopic Hydrodistention
At a minimum 2 weeks after initiating behavioral/pharmacologic therapy, each patient was evaluated via a standardized cystoscopic protocol. After a successful general anesthesia, a 21F cystoscope was inserted per urethra and the bladder was surveyed. The bladder was then filled to capacity with sterile water via gravity irrigation (100 cm H20 above pubic symphysis). The bladder was then emptied and the bladder surveyed again with the cystoscope. Mucosal glomerulization and Hunner’s ulcers were specifically recorded, if present. Hydrodistension was repeated three times at bladder capacity. Bladder capacity under anesthesia for the three distensions was averaged and recorded as maximum bladder capacity.
Outcome Assessment
The
severity of PBS/IC symptoms before and after interventions was assessed
using the validated O’Leary-Sant Interstitial Cystitis questionnaire.
This robust questionnaire is an 8 item form divided into two domains assessing
symptom severity (Symptom Index - 4 questions) and the impact of interstitial
cystitis (IC) on daily life (Problem Index - 4 questions). Each question
is scored by the patient with higher numbers in each domain representing
greater severity and impact. Maximum Symptom and Problem Index scores
were 20 and 16, respectively (6). The patients were given the O’Leary-Sant
questionnaire at initial clinical presentation. The questionnaires were
again administered after 1 month of behavioral and pharmacologic therapy
treatment. Patients returned within 2 months after hydrodistension for
questionnaire completion and were scheduled for return visits every three
to six months afterwards for questionnaire completion. Voiding diaries
were not utilized.
Paired t-tests and ANOVA were used to analyze
the data. Statistical significance was defined as p < 0.05. All tests
were performed with statistical software SPSS v13.0 (SPSS, Inc., Chicago,
IL, USA).
RESULTS
Twenty-five
patients were prospectively enrolled in the study between July 2004 and
August 2006. Patients had PBS/IC symptoms for a median 12 months (range
3-60) prior to presentation at our institution. Eighteen (72%) completed
the pilot multimodal treatment protocol and returned questionnaires for
evaluation. Of the 7 patients who were excluded prior to analysis, 3 patients
did not wish to continue the behavioral therapy and did not wish to pursue
hydrodistension. Two patients were lost to follow-up prior to hydrodistension,
1 patient was excluded due to newly diagnosed pelvic endometriosis during
the study and 1 patient was excluded after transitional cell carcinoma
of the bladder was found during the endoscopic hydrodistension.
The median age at presentation for these
patients was 36.3 years (SD 15.9) and the most common presenting symptom
was urinary urgency (18 patients), followed by urinary frequency (17 patients)
and pelvic pain (16 patients). Associated comorbidities, based on diagnosis
from qualified treating physicians, included depression (67%), irritable
bowel syndrome (28%), anxiety (28%), inflammatory bowel disease (17%),
and fibromyalgia (17%).
All patients completing the protocol reported good compliance with the
behavioral fluid management/timed voiding/Kegel exercise regimen and there
were no marked protocol deviations. Seventeen patients remained on Urised
(94%), and 16 on hydroxizine and macrodantin (89%) throughout the study.
Patients withdrawing from the suggested medications cited gastrointestinal
irritation (urised - 1 patient, macrodantin - 1 patient) and dizziness
(hydroxyzine - 1 patient) as precipitating factors. Three patients reported
Elmiron usage for greater than 6 months prior to initiation of treatment
and were maintained on the prescribed medication.
All 18 patients underwent hydrodistention
under anesthesia at a mean of 2.1 months (SD 1.3) from the initiation
of behavioral and pharmacologic therapy. Significant glomerulations during
cystoscopy were seen in 17 patients (94%). No Hunner’s ulcers were
identified. The mean maximum bladder capacity under anesthesia was 836
milliliters (SD 154). All patients tolerated the hydrodistension and were
restarted on their exact behavioral and pharmacologic regimens post-operatively.
There were no adverse events reported from the hydrodistension.
Mean follow-up for patients completing the protocol was 10.2 months (CI
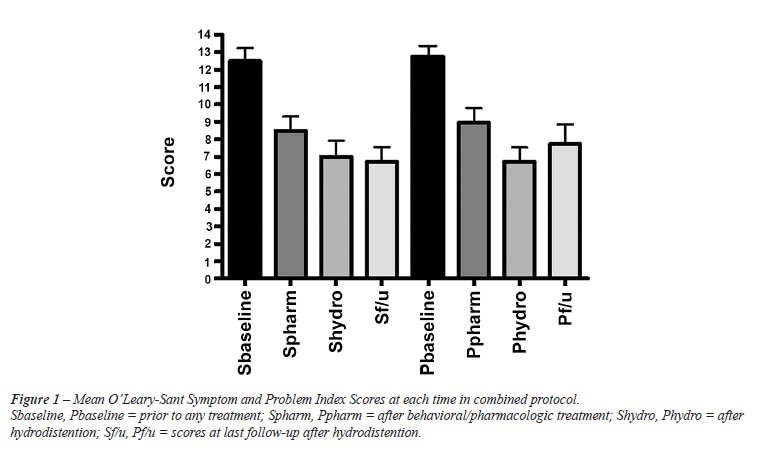
95% [5.7, 14.7]). The mean initial symptom and problem index scores prior
to initiating the combined protocol were 12.5 (CI 95% [10.9, 14.1]) and
12.7 (CI [11.4, 14.1]). After behavioral modification and pharmacologic
therapy but prior to hydrodistension, 18 patients completed questionnaires.
Mean symptom and problem index scores were 8.5 ([6.8, 10.2]) and 8.9 ([7.2,
10.7]). At 2 months after hydrodistension, 14 patients completed questionnaires
and mean symptom and problem index scores were 7.0 ([5.0, 9.0]) and 6.7
([5.0, 8.5]), respectively. At last follow-up after hydrodistension, 7
patients had available data. Mean symptom and problem indexes for these
patients were 6.7 ([4.7, 8.8]) and 7.7 ([4.9, 10.5]). Changes in symptom
(p < 0.001) and problem index scores (p < 0.001) from baseline were
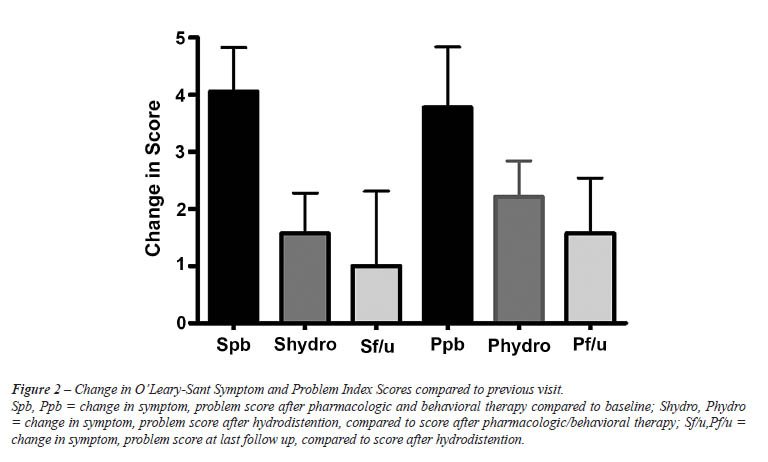
significant. Data are summarized in Figure-1. The greatest change in problem
and symptom scores was seen in the interval between baseline and of behavioral/pharmacologic
therapy (Figure-2).
COMMENTS
In
this study, we prospectively evaluated symptomatic PBS/IC patients treated
with a unique pilot multimodal program, consisting of behavioral, pharmacologic,
and endoscopic hydrodistension therapy. We demonstrated significant statistical
improvement in QOL scores over baseline after initiating the behavioral/pharmacologic
therapy, maintenance of this improvement after hydrodistension and sustainable
relief for months afterwards. This is the first study, to our knowledge,
to demonstrate efficacy for a prospectively evaluated multimodal behavioral,
pharmacologic, endoscopic protocol. Based on these initial results, a
larger randomized trial investigating the efficacy of the individual protocol
components appears justified.
Literature on combined protocols for treating
PBS/IC is generally limited (7-9). Consequently, we can not compare our
outcomes to other multimodal therapies.
However, physicians commonly use multiple
modalities for treating PBS/IC. In Rovner et al.’s review of the
Interstitial Cystitis Data Base (ICDB), these authors found over 180 different
types of PBS/IC treatments, with 21%, 34%, and 28% of women treated via
single-mode, a combination of two, and three or more different types of
therapies, respectively (10). Consequently, our pilot study suggests more
research should be performed to prospectively investigate the efficacy
of multimodal approaches.
The results of our multimodal therapy compare
favorably with single modality therapies. Pentosanpolysulfate (PPS) therapy,
the oral heparin analogue, showed a 26% subjective improvement in a placebo
controlled multi-center trial (11). Amitriptyline therapy has a similar
efficacy in the literature. In one of the largest prospective, randomized,
placebo-controlled, double-blind study, the mean total symptom score decreased
from 26.9 to 18.5 (31%) in the amitriptyline group compared with 27.6
- 24.1 in the placebo group, p = 0.005, (12). Initial results from our
multimodality protocol also appear at least as effective as intravesical
therapy, another mainstay therapy for PBS/IC (13). Since neither PPS,
amitriptyline, nor intravesical therapy has gained universal use among
practitioners, our protocol, if validated, may provide further treatment
options for frustrated physicians and patients.
We selected modalities for our protocol
based on ease of implementation and historical reports of efficacy. At
the first visit, we begin with behavioral modification that focuses on
timed voiding and fluid management because there is some evidence that
these strategies may play some role in symptom reduction. Chaikan et al.
had 42 patients with refractory symptoms undergo fluid management/timed
voiding and showed an decrease in urinary frequency from 17 voids per
day to 8 voids per day (14). Although true evidence based evaluations
of these behavioral measures are lacking in the literature, Whitmore suggests
that behavioral changes may, at minimum, empower the patient and improve
coping mechanisms (15). We noted almost universal compliance and self-reported
improvement with timed voiding, fluid restriction, food exclusion and
pelvic floor exercises and strongly concur with this suggestion.
We prescribe three medications, hydroxyzine,
macrobid, and urised (methenamine, methylene blue, phenyl salicylate,
benzoic acid, atropine sulfate, hyoscyamine) as part of our multimodality
plan. We selected hydroxyzine based on the theory that it is a mast cell
stabilizer and thus may play an important role in mediating the inflammatory
process observed in PBS/IC (16). As an adjunct to hydroxyzine, we also
utilized macrodantin. Although not commonly considered first line therapies
for PBS/IC, some studies suggest that some cases of PBS/IC may be due
to dormant microbes or an infectious etiology (17). Finally, we utilize
Urised as an antispasmodic. Little information is available on antispasmodic
use for treating PBS/IC, although Hill recently reported that patients
taking phenothiazide consistently reported PBS/IC symptom reduction while
on this medication (18). This observation matches our own clinical experience.
As the final treatment arm of our multimodal
therapy plan, we performed endoscopic hydrodistension under anesthesia
a mean 2.1 months after starting the behavioral and pharmacologic treatments.
Although somewhat controversial, some studies suggest that endoscopic
hydrodistention has (short term) efficacy in the treatment of PBS/IC symptoms.
Ottem & Teichman, retrospectively reviewed 84 consecutive PBS/IC patients
treated with hydrodistension and found over 50% reported symptomatic improvement
2 months after the procedure (19). Unfortunately, however, it is difficult
to generalize results from hydrodistension studies due to variance in
techniques and outcome reporting. In our study, patients showed significant,
if small, improvement in quality of life after undergoing hydrodistension.
Consequently, we believe adding this procedure to the regimen of behavioral
and pharmacologic therapy yields benefit for the symptomatic PBS/IC patient.
As with any observational study examining
PBS/IC treatments, this study has limitations. We recognize that the PBS/IC
subjects in our study may not be representative of the general PBS/IC
population since the general population is difficult to standardize. For
example, we utilized the PBS/IC Study Data Base recommendations as inclusion
and exclusion criteria and the mean bladder capacity in our study was
836 mL. In contrast, studies using the NIDDK inclusion/exclusion criteria
for diagnosing PBS/IC would have excluded all patients with a bladder
capacity greater than 350 mL. Furthermore, none of our patients had mucosal
ulcerations on cystoscopic examination. In other studies, the prevalence
of mucosal ulceration ranges as high as 20% (20).
Outcomes in our study were measured via
change in the validated O’Leary-Sant IC questionnaire. We chose
this outcome measure because voiding diaries hold too much internal variability
to be reliable measures for a small efficacy study. Currently, there are
three published PBS/IC quality of life questionnaires: the O’Leary-Sant
IC Symptom Index and IC Problem Index, the University Of Wisconsin IC
Scale, and the Pelvic Pain and Urgency/Frequency Scale. In theory, investigators
employing different questionnaires may yield interpretations of outcomes.
Although data from this study is limited
to 18 patients, the initial data from this pilot study is encouraging.
Significant statistical improvement in PBS/IC quality of life scores was
noted for these patients both after starting behavior/pharmacologic and
after hydrodistension. Despite this clinically significant improvement,
we recognize that these observations could be confounded by a potential
placebo effect and by spontaneous remission of PBS/IC symptoms. In general,
placebo controlled trials are lacking in PBS/IC research and the placebo
effect can be difficult to quantify even in randomized controlled PBS/IC
trials. Spontaneous remission rates certainly should be considered when
interpreting outcomes from any PBS/IC study, even though the epidemiology
of spontaneous remission is poorly understood or studied. Since there
is no solid research documenting remission rates or timeframes, we can
not speculate on how our data was effected other than comment that outcomes
appear durable in our limited data. Further investigation with blinded,
randomized trials is needed to better understand any potential placebo
effect.
CONCLUSION
This pilot trial suggests that symptomatic PBS/IC patients treated with a multimodal treatment plan consisting of behavioral, pharmacologic, and endoscopic therapy demonstrate significant, progressive, and durable improvement. A placebo controlled trial with longer follow up is needed to validate these findings.
ACKNOWLEDGEMENTS
Robert S. Hanley and John T. Stoffel contributed equally to the manuscript and share first authorship.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al.: The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21: 167-78.
- Koziol JA, Clark DC, Gittes RF, Tan EM: The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993; 149: 465-9.
- Kelada E, Jones A: Interstitial cystitis. Arch Gynecol Obstet. 2007; 275: 223-9.
- Simon LJ, Landis JR, Tomaszewski JE, Nyberg LM: The Interstitial Cstitis Database (ICDB) Study. In: Interstitial Cystitis, Sant GR (ed.). Philadelphia, Lippincott-Raven. 1997; pp. 17-24.
- Gillespie L: Interstitial Cystitis and Diet. In: Interstitial Cystitis, Sant GR (ed.). Philadelphia, Lippincott-Raven. 1997, pp. 111.
- O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarich-Kroll J: The interstitial cystitis symptom index and problem index. Urology. 1997; 49(5A Suppl): 58-63.
- Liu HT, Kuo HC: Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007; 70: 463-8.
- Baykal K, Senkul T, Sen B, Karademir K, Adayener C, Erden D: Intravesical heparin and peripheral neuromodulation on interstitial cystitis. Urol Int. 2005; 74: 361-4.
- Dell JR, Butrick CW: Multimodal therapy for painful bladder syndrome/interstitial cystitis. J Reprod Med. 2006; 51(3 Suppl): 253-60.
- Rovner E, Propert KJ, Brensinger C, Wein AJ, Foy M, Kirkemo A, et al.: Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology. 2000; 56: 940-5.
- Mulholland SG, Hanno P, Parsons CL, Sant GR, Staskin DR: Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology. 1990; 35: 552-8.
- van Ophoven A, Pokupic S, Heinecke A, Hertle L: A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004; 172: 533-6.
- Dawson TE, Jamison J: Intravesical treatments for painful bladder syndrome/ interstitial cystitis. Cochrane Database Syst Rev. 2007; 17: CD006113.
- Chaiken DC, Blaivas JG, Blaivas ST: Behavioral therapy for the treatment of refractory interstitial cystitis. J Urol. 1993; 149: 1445-8.
- Whitmore KE: Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994; 21: 121-30.
- Theoharides TC, Sant GR: Hydroxyzine therapy for interstitial cystitis. Urology. 1997; 49(5A Suppl): 108-10.
- Domingue GJ, Ghoniem GM, Bost KL, Fermin C, Human LG: Dormant microbes in interstitial cystitis. J Urol. 1995; 153: 1321-6. Erratum in: J Urol 1996; 155: 298.
- Hill JR, Isom-Batz G, Panagopoulos G, Zakariasen K, Kavaler E: Patient perceived outcomes of treatments used for interstitial cystitis. Urology. 2008; 71: 62-6.
- Ottem DP, Teichman JM: What is the value of cystoscopy with hydrodistension for interstitial cystitis? Urology. 2005; 66: 494-9.
- Koziol JA, Adams HP, Frutos A: Discrimination between the ulcerous and the nonulcerous forms of interstitial cystitis by noninvasive findings. J Urol. 1996; 155: 87-90.
____________________
Accepted
after revision:
March 6, 2009
_______________________
Correspondence
address:
Dr. John T. Stoffel
Departmentof Urology
Lahey Clinic
41 Mall Road
Burlington, MA, 01805, USA
E-mail: john.t.stoffel@lahey.org