Experimental
Model of Human Corpus Cavernosum Smooth Muscle Relaxation
(
Download pdf )
doi: 10.1590/S1677-5538201000400012
Basic and Translational Urology
Rommel P. Regadas, Maria E. A. Moraes, Francisco J. C. Mesquita, Joao B. G. Cerqueira, Lucio F. Gonzaga-Silva
Department of Surgery (RPR, JBGC, LFGS, FJCM) and Department of Pharmacology (MEAM), School of Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil
ABSTRACT
Purpose:
To describe a technique for en bloc harvesting of the corpus cavernosum,
cavernous artery and urethra from transplant organ donors and contraction-relaxation
experiments with corpus cavernosum smooth muscle.
Materials and Methods: The corpus cavernosum
was dissected to the point of attachment with the crus penis. A 3 cm segment
(corpus cavernosum and urethra) was isolated and placed in ice-cold sterile
transportation buffer. Under magnification, the cavernous artery was dissected.
Thus, 2 cm fragments of cavernous artery and corpus cavernosum were obtained.
Strips measuring 3 x 3 x 8 mm3 were then mounted vertically in an isolated
organ bath device. Contractions were measured isometrically with a Narco-Biosystems
force displacement transducer (model F-60, Narco-Biosystems, Houston,
TX, USA) and recorded on a 4-channel Narco-Biosystems desk model polygraph.
Results: Phenylephrine (1µM) was used
to induce tonic contractions in the corpus cavernosum (3 - 5 g tension)
and cavernous artery (0.5 - 1g tension) until reaching a plateau. After
precontraction, smooth muscle relaxants were used to produce relaxation-response
curves (10-12M to 10-4 M). Sodium nitroprusside was used as a relaxation
control.
Conclusion: The harvesting technique and
the smooth muscle contraction-relaxation model described in this study
were shown to be useful instruments in the search for new drugs for the
treatment of human erectile dysfunction.
Key
words: penis; cavernous artery; penile erection; experimental;
erectile dysfunction
Int Braz J Urol. 2010; 36: 490-6
INTRODUCTION
Erectile
dysfunction (ED) affects approximately 150 million people worldwide. The
prevalence of ED in Brazil is high: more than 40% of Brazilian men between
40 and 70 years of age suffer from ED and more than a million new cases
are registered annually (1,2).
Although phosphodiesterase type-5 (PDE-5) inhibitors have revolutionized
the treatment of erectile dysfunction, many patients, mostly those with
endothelial dysfunction (56% of cases), do not benefit from this form
of therapy (3).
At present many studies are being carried
out using nitric oxide (NO) donors, guanylyl cyclase activators (both
soluble intracellular and membrane-bound isoforms), ion channel agonists
and RhoA-kinase inhibitors in order to formulate new drugs with different
mechanisms of action to treat this patient population (4,5).
The vast majority of these studies employ rat and rabbit corpus cavernosum
due to the difficulty in obtaining samples of human tissue (5-7). However,
at our Urology Service, experimental studies on ED have been in progress
since 2004 using human corpus cavernosum tissue from organ donors.
The purpose of the present study was to
provide a detailed description of the technique used for en bloc harvesting
of the corpus cavernosum, cavernous artery and urethra of transplant organ
donors and the methods used in contraction-relaxation experiments with
corpus cavernosum smooth muscle.
MATERIALS AND METHODS
All
study protocols were previously approved by the Human Subjects Research
Ethics Committee of the Federal University of Ceará and by the
National Research Ethics Committee of the Brazilian Ministry of Health.
Following authorization from the family,
human corpus cavernosum was obtained from cadaver donors (< 40 years)
during surgery for organ transplantation.
After removal of the heart, liver and kidneys and through the same incision
(xiphoid pubic), the corpus cavernosum was located above the pubic symphysis
by digital hypodermic approach. The corpus cavernosum was dissected to
the point of attachment with the ischiopubic ramus (crus penis) (Figure-1).
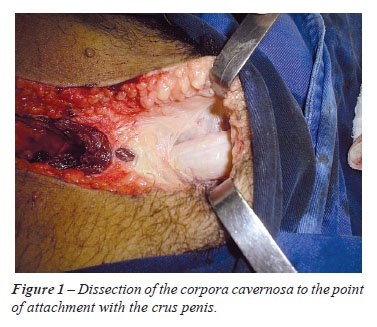
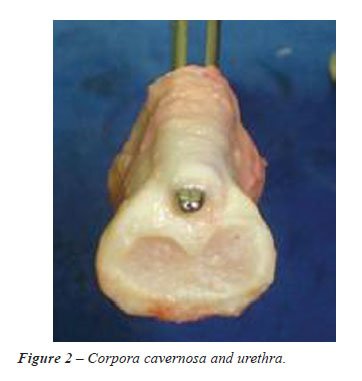
A 3 cm segment including the corpus cavernosum
and urethra was isolated en bloc (Figure-2). No additional external incision
was made at the end of procedure. Subsequently, the tissues were placed
in ice-cold sterile transportation buffer (Collins solution) and processed
within 1 hour after collection.
The samples were processed under stereoscopic
magnification. The entire cavernous artery in the center of the corpus
cavernosum was dissected and isolated from the surrounding cavernous tissues
(Figure-3). Then cavernous tissues were separated from connective tissues
and the tunica albuginea. Thus, 2 cm fragments of each cavernous artery
and corpus cavernosum were obtained.

The corpus cavernosum fragments were cut
into strips measuring approximately 3 x 3 x 8 mm3 and mounted vertically
under 1g resting tension. The cavernous artery was cut into 5 mm rings
and mounted horizontally under 0.2g resting tension. The tissues were
maintained in 5 mL organ chambers containing Krebs-Henseleit medium composed
of 114.6 mM NaCl, 4.96 mM KCl, 1.3 mM MgSO4, 2.0 mM CaCl2, 1.23 mM NaH2PO4,
25 mM NaHCO3 and 3.6 mM glucose, enriched with 10 µM guanethidine
and 10 µM indomethacin (pH 7.4, 37ºC, gassed with 5% CO2 and
95% O2).
The tissues were allowed to equilibrate
for 90 min with washing at 15 min intervals. The tension was measured
by an isometric transducer (F-60 Narco-Biosystems connected to a 4-channel
desk model polygraph) (Figure-4).
One micromole phenylephrine was added to
the baths to obtain 60-70% submaximal smooth muscle contractions. Subsequently,
concentration-response curves (10-8M to 10-2 M) to smooth-muscle relaxants
or sodium nitroprusside (SNP), a nitric oxide donor, were plotted to check
for endothelial functional integrity.

RESULTS
Phenylephrine
(1µM) was used to induce tonic contractions in the corpus cavernosum
(3 - 5g tension) and cavernous artery (0.5 - 1g tension) until reaching
a plateau. After precontraction, smooth muscle relaxants were used to
produce relaxation-response curves (10-12M to 10-4 M). SNP was used as
a relaxation control.
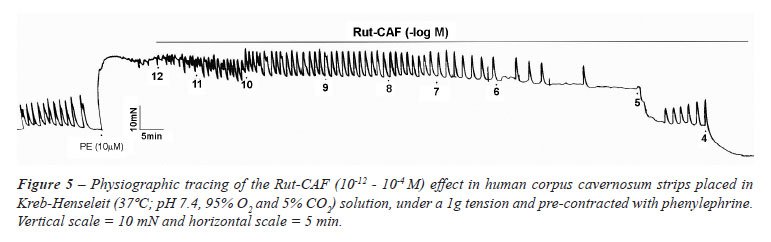
A number of chemical substances have been
used in our laboratory to induce smooth muscle relaxation, including Ru[(NH3)4(caffeine)(NO)]C13,
a nitric oxide donor. It completely relaxes the human corpus cavernosum
and cavernous artery achieving an Emax of 100% and an EC50 of 6.4 ±
0.14 (Figure-5).
Using preparations of corpus cavernosum
with intact endothelium from human donors under 40 with no history of
erectile dysfunction or cardiovascular risk factors (e.g. diabetes, hypertension
and dyslipidemias), this physio-pharmacological model proved to be an
attractive instrument in the search for new drugs for the treatment of
human erectile dysfunction.
COMMENTS
PDE-5
inhibitors have revolutionized the treatment of erectile dysfunction.
However, many patients with ED also suffer from endothelial dysfunction
(56%) and are therefore unresponsive to this class of drugs (3).
Endothelial dysfunction is often observed
in patients with comorbidities such as arterial hypertension and diabetes
mellitus. It is characterized by a deficiency in the endogenous production
of NO (8).
In true ED, diabetes, hypertension, and
dyslipidemia (components of the metabolic syndrome) tend to be associated
with endothelial dysfunction. ED has also been reported to be a marker
for cardiovascular arterial disease (9).
The search for new drugs capable of increasing
the availability of endogenous NO has been a considerable challenge. Several
experimental models have been used over the past decades based on rat,
rabbit and human corpus cavernosum (6,7,10,11).
Using corpus cavernosum in vivo and other
tissues (e.g. platelets) from species such as rats, rabbits and humans,
Peng Wang et al. (12) concluded that, in spite of similar kinetics and
enzymatic features, different PDEs have different sensitivities to inhibitors.
This should be taken into account when working with experimental models
of this type.
Our experimental model employed healthy
corpus cavernosum tissues from young cadaver donors killed by trauma or
stroke in order to minimize the concern about distortion of results caused
by sample tissues of poor condition.
In contrast, in a study using a similar
human corpus cavernosum model for the evaluation of the effect of sildenafil
on enzymatic PDE inhibition and consequent smooth muscle relaxation, samples
were obtained from patients with ED during surgery for penile prosthesis
implantation, so it seems likely that in this case most of the subjects
presented endothelial injury to some degree (6).
Another concern in this field of research is the availability of tissues
to perform the experiments. In Ceará, eight organ transplantations
are carried out every month, making it possible to complete studies without
major interruptions.
Seidler et al. (4) worked on a similar model
using corpus cavernosum donated by patients undergoing sex reassignment
surgery as treatment for transsexualism and gender identity disorder.
In spite of the good condition of the tissues, the small number of men
submitting to this type of procedure limits the possibility of collecting
sufficient tissue for experimental work.
The present paper presents a comprehensive
model for harvesting human corpus cavernosum tissues and for carrying
out smooth muscle relaxation experiments in vivo. Healthy human corpus
cavernosum is removed from cadaver donors and subjected to experiments
in isolated baths. The technique allows to dissect and isolate the corpus
cavernosum, cavernous artery and urethra.
The importance of the technique lies in that it makes it possible to test
a range of new drugs, including stable NO donors, guanylyl cyclase activators
and RhoA-kinase inhibitors, on smooth muscle corpus cavernosum, penile
arteries and urethra (7,12-15).
CONCLUSION
This experimental model involves the dissection, harvesting, isolation and conservation of the human corpus cavernosum, cavernous artery and urethra under ideal conditions along with the accompanying physio-pharmacological studies. The feasibility and reproducibility of the model makes it an attractive instrument in the search for new drugs for the treatment of human erectile dysfunction.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Moreira ED Jr, Bestane WJ, Bartolo EB, Fittipaldi JA: Prevalence and determinants of erectile dysfunction in Santos, southeastern Brazil. Sao Paulo Med J. 2002; 120: 49-54.
- Moreira ED Jr, Lisboa Lôbo CF, Villa M, Nicolosi A, Glasser DB: Prevalence and correlates of erectile dysfunction in Salvador, northeastern Brazil: a population-based study. Int J Impot Res. 2002; 14(Suppl 2): S3-9.
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB: Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151: 54-61.
- Seidler M, Uckert S, Waldkirch E, Stief CG, Oelke M, Tsikas D, et al.: In vitro effects of a novel class of nitric oxide (NO) donating compounds on isolated human erectile tissue. Eur Urol. 2002; 42: 523-8.
- Lopes LFG, Wieraszko AY, El-Sherif, Clarke MJ: D-trans-labilization of Nitric Oxide in Ru-II Complexes by C-bound Imidazoles. Inorg-Chim Acta 2001; 312: 15-22.
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM: Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998; 159: 2164-71.
- Prieto D, Rivera L, Recio P, Rubio JL, Hernández M, García-Sacristán A: Role of nitric oxide in the relaxation elicited by sildenafil in penile resistance arteries. J Urol. 2006; 175: 1164-70.
- Rendell MS, Rajfer J, Wicker PA, Smith MD: Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999; 281: 421-6.
- Palumbo PJ: Metabolic risk factors, endothelial dysfunction, and erectile dysfunction in men with diabetes. Am J Med Sci. 2007; 334: 466-80.
- Thompson CS, Mumtaz FH, Khan MA, Wallis RM, Mikhailidis DP, Morgan RJ, et al.: The effect of sildenafil on corpus cavernosal smooth muscle relaxation and cyclic GMP formation in the diabetic rabbit. Eur J Pharmacol. 2001; 425: 57-64.
- Angulo J, Cuevas P, Moncada I, Martín-Morales A, Allona A, Fernández A, et al.: Rationale for the combination of PGE(1) and S-nitroso-glutathione to induce relaxation of human penile smooth muscle. J Pharmacol Exp Ther. 2000; 295: 586-93.
- Wang P, Wu P, Myers JG, Stamford A, Egan RW, Billah MM: Characterization of human, dog and rabbit corpus cavernosum type 5 phosphodiesterases. Life Sci. 2001; 68: 1977-87.
- Martinez AC, García-Sacristán A, Rivera L, Benedito S: Biphasic response to histamine in rabbit penile dorsal artery. J Cardiovasc Pharmacol. 2000; 36: 737-43.
- Matsumoto A, Morita T, Kondo S: Alpha-adrenoceptor-mediated penile erection in dogs: in vivo and in vitro observations. J Smooth Muscle Res. 2000; 36: 169-79.
- Andersson KE, Gratzke C: Pharmacology of alpha1-adrenoceptor antagonists in the lower urinary tract and central nervous system. Nat Clin Pract Urol. 2007; 4: 368-78.
____________________
Accepted after revision:
January 20, 2010
_______________________
Correspondence address:
Dr. Rommel Prata Regadas
Dr. Ratisbona, 208, Fatima
Fortaleza, Ceará, 60411-220, Brazil
Fax: + 55 85 3366-8064
E-mail: rommelregadas@ig.com.br
EDITORIAL COMMENT
Penile
erection is a complex neurovascular event that relies on vasodilatation
of erectile tissues due to neuronal and endothelial derived nitric oxide
(NO) released by activation of parasympathetic nerves on sexual stimulation
of the cavernous endothelial lining (1).
This sexual stimulus brings about blood
flow into the corpus cavernosum and the consequent penile rigidity is
maintained by means of a veno-occlusive mechanism.
This is enabled by the particular micro-architecture
of the corpus cavernosum, which consents a sophisticated hemodynamic system.
Otherwise, the tunica albuginea plays a key role in the erectile function.
Being rich in elastic fibers it is able
to resist overstretching of the corpus at raised levels of intracavernous
pressure, compressing the trans-albugineal effluent veins, as well providing
on inextensible protective structure to the arteriole and to the intracavernous
nerves.
This function is possible due to its structure
made of collagenic fibers linked by elastic fiber bridges (2,3).
Therefore, it is very important to keep
its integrity to maintain its fundamental role in the erectile mechanism.
The presence of structural disorders like
an excessive collagen deposition gives rise to the formation of a plaque,
fibrotic first and then calcified, as can be found in Peyronie’s
disease.
Moreover, there is a significant decrease of elastic fiber concentration
as well in these patients affected by induration penis plastica (4).
Similar changing were found in patients
who underwent radical prostatectomy, where the trabecular elastic fibers
and smooth muscle fibers were decreased and collagen content was significantly
increased (5).
As age advances the gonadal steroid hormones,
and in particular, testosterone production decreases (6), nerve conduction
slows down and the efficiency of the vascular microcirculation of the
penis is reduced.
Androgens are essential for the development,
growth and maturation of erectile tissues, acting on the hemostatis in
the corpora cavernosum, regulating the growth of smooth muscle and protein
synthesis of the connective tissues.
Therefore, a decrease in their production
could give rise to the switch from elastic fibers to collagen fibers,
which is the basis of cavernosal fibrosis (7,8).
Recent studies have shown that testosterone
also regulates the expression of phosphodiesterasis type 5 (PDE5) (9).
It is known that erectile dysfunction (ED)
affects 150 million people worldwide.
Until few years ago, it was thought that
90% of ED had a psychogenetic etiology.
Moreover, further neurophysiological, hemodynamical
and pharmacological studies have helped us to understand better the complex
biochemical and micro-anatomical mechanism of the erectile function, showing
us that 50% of ED has an organic etiology (10).
On the other hand, even psychogenetic ED
could be the consequence of an increase of adrenergic stimulation and
having itself an organic origin (11).
The past 20 years have witnessed remarkable
changes in the treatment of ED.
The emergence and the success of PDE5 inhibitors
as effective therapy for erectile dysfunction is remarkable considering
the intent behind the development of the original compound: initially
designed as an antianginal agent, it quickly became apparent that the
first PDE5 inhibitor on the market, sildenafil, displayed erectogenesis
as a side effect, and the drug was soon recognized as a potential revolutionary
treatment for ED..
Furthermore, sildenafil has been shown to
prevent the progression of fibrosis of the corpus cavernosum in prostatectomized
patients. Its efficacy seems to result from an anti-proliferative effect
exerted on fibroblasts (12).
It is known that PDE-5 inhibitors have revolutionized
the treatment of erectile dysfunction and changed the life of million
people worldwide.
However there still a high percentage of
patients with ED that are also affected by endothelial dysfunction (56%)
and subsequently they are unresponsive to this class of drugs (13).
Although already extensively studied, NO
donors continue to be an important topic as regards ED.
Many studies have been carried out to find
new NO donors or new guanylyl cyclase activators to try to find new drugs
to treat these patients who are non-responders to PDE-5 inhibitors (14).
The present work shows us a model for harvesting
human corpus cavernosum tissues and for making smooth muscle relaxation
experiments in vivo.
The healthy corpus cavernosum taken from
young cadaver donors killed by trauma or stroke offer tissues in good
condition.
In the literature, we have not found a similar
approach due the difficulty to obtain samples of human tissues.
With this technique is possible to test
new drugs, like NO donors, guanylyl cyclase activators and RohA-Kinase
inhibitors on human smooth muscle tissues in vivo rather then using corpus
cavernosum in vivo from animals like rats, or rabbits as it has been performed
by Pen Wang et al. (15).
Finally, this harvesting technique and smooth
muscle contraction-relaxation model could be a very useful instrument
to help us to find new drugs to treat ED.
REFERENCES
- Andersson KE, Wagner G: Physiology of penile erection. Physiol Rev. 1995; 75: 191-236.
- Iacono F, Barra S, de Rosa G, Boscaino A, Lotti T: Microstructural disorders of tunica albuginea in patients affected by impotence. Eur Urol. 1994; 26: 233-9.
- Iacono F, Barra S, Lotti T: Elastic fibre concentration in the tunica albuginea of corpora cavernosa and nocturnal tumescence monitoring. Int J Impot Res. 1995; 7: 63-70.
- Iacono F, Barra S, De Rosa G, Boscaino A, Lotti T: Microstructural disorders of tunica albuginea in patients affected by Peyronie’s disease with or without erection dysfunction. J Urol. 1993; 150: 1806-9.
- Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V: Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005; 173: 1673-6.
- Traish A, Kim N: The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med. 2005; 2: 759-70.
- Traish AM, Guay AT: Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med. 2006; 3: 382-404; discussion 404-7.
- Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW: A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001; 13: 73-81.
- Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, Marini M, et al.: Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004; 145: 2253-63. Erratum in: Endocrinology. 2004; 145: 3152.
- Kaiser FE: Erectile dysfunction in the aging man. Med Clin North Am. 1999; 83: 1267-78.
- Iacono F, Barra S, Lotti T: Evaluation of penile deep arteries in psychogenic impotence by means of duplex ultrasonography. J Urol. 1993; 149: 1262-4.
- Iacono F, Prezioso D, Somma P, Chierchia S, Galasso R, Micheli P: Histopathologically proven prevention of post-prostatectomy cavernosal fibrosis with sildenafil. Urol Int. 2008; 80: 249-52.
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB: Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151: 54-61.
- Seidler M, Uckert S, Waldkirch E, Stief CG, Oelke M, Tsikas D, et al.: In vitro effects of a novel class of nitric oxide (NO) donating compounds on isolated human erectile tissue. Eur Urol. 2002; 42: 523-8.
- Wang P, Wu P, Myers JG, Stamford A, Egan RW, Billah MM: Characterization of human, dog and rabbit corpus cavernosum type 5 phosphodiesterases. Life Sci. 2001; 68: 1977-87.
Dr.
Fabrizio Iacono
Dr. Domenico Taglialatela & Dr. Antonio Ruffo
Department Urology
University “Federico II”
Naples, Italy
E-mail: fiacon@tin.it