Diminution
of Oxalate Induced Renal Tubular Epithelial Cell Injury and Inhibition
of Calcium Oxalate Crystallization in vitro by Aqueous Extract of Tribulus
terrestris
(
Download pdf )
doi: 10.1590/S1677-55382010000400011
Basic and Translational Urology
A. Aggarwal, S. Tandon, S. K. Singla, C. Tandon
Department of Biotechnology and Bioinformatics (AA, ST, CT), Jaypee University of Information Technology, Waknaghat, Solan, India and Department of Biochemistry (SKS), Panjab University, Chandigarh, India
ABSTRACT
Purpose:
Recurrence and persistent side effects of present day treatment for urolithiasis
restrict their use, so an alternate solution, using phytotherapy is being
sought. The present study attempted to evaluate the antilithiatic properties
of Tribulus terrestris commonly called as “gokhru” which is
often used in ayurveda to treat various urinary diseases including urolithiasis.
Materials and Methods: The activity of Tribulus
terrestris was investigated on nucleation and the growth of the calcium
oxalate (CaOx) crystals as well as on oxalate induced cell injury of NRK
52E renal epithelial cells.
Results: Tribulus terrestris extract exhibited
a concentration dependent inhibition of nucleation and the growth of CaOx
crystals. When NRK-52E cells were injured by exposure to oxalate for 72
h, Tribulus terrestris extract prevented the injury in a dose-dependent
manner. On treatment with the different concentrations of the plant, the
cell viability increased and lactate dehydrogenase release decreased in
a concentration dependent manner.
Conclusion: The current data suggests that
Tribulus terrestris extract not only has a potential to inhibit nucleation
and the growth of the CaOx crystals but also has a cytoprotective role.
Our results indicate that it could be a potential candidate for phytotherapy
against urolithiasis.
Key
words: phytotherapy; urolithiasis; calcium oxalate; NRK 52E,
Tribulus terrestris
Int Braz J Urol. 2010; 36: 480-9
INTRODUCTION
Nephrolithiasis
is common, affecting up to 10% of the population at some point during
their lifetime (1). Calcium-containing stones are the most commonly occurring
to an extent of 75-90% followed by magnesium ammonium phosphate (Struvite)
to an extent of 10-15%, uric acid 3-10% and cystine 0.5-1% (2). Calcium
oxalate stones are found in two different varieties, calcium oxalate monohydrate
(COM) or Whewellite, and calcium oxalate dihydrate (COD) or Weddellite.
COM, the thermodynamically most stable form, is observed more frequently
in clinical stones than COD and it has a greater affinity for renal tubular
cells, thus responsible for the formation of stones in the kidney (3).
Various authors have suggested the role
of crystal induced cell injury in the development of kidney stones by
providing the sites for crystal attachment and retention within the kidneys
(4,5).
Oxalate, a metabolic end product and a major constituent of the majority
of renal stones, has been shown to be toxic to renal epithelial cells
of cortical origin (6). It has been observed that exposure of renal epithelial
cells to oxalate which is a constituent of most kidney stones leads to
a disruption of the normal activities of the renal epithelial cells such
as altered membrane surface properties and cellular lipids, changes in
gene expression, disruption of mitochondrial function, formation of reactive
oxygen species and decreased cell viability (7).
Various mechanisms have been proposed to
explain crystal retention (8). As a result of crystal growth and agglomeration,
particles may be formed that are too large to freely pass the renal tubules.
Alternatively, relatively small crystals could be retained by adhering
to the surface of the urothelial lining and then increase in size (8).
The surgical methods available to treat
kidney stones like extracorporeal shock wave lithotripsy have serious
side effects. Therefore, it is worthwhile to look for an alternative for
the management of urolithiasis. Many medicinal plants have been employed
during ages to treat urinary stones though the rationale behind their
use is not well established through systematic and pharmacological studies,
except for some composite herbal drugs and plants (9-12). Plant medicines
are in great demand both in the developed as well as developing countries
for primary health care because of their wide range of biological and
medicinal activities, higher safety margin and low cost.
Fruits of Tribulus terrestris (Zygophyllaceae)
locally named as “gokhru” in India are commonly used in folklore
to treat urolithiasis. So far, its diuretic properties have been documented
in literature and it is actively used in various drug formulations of
kidney stone treatments.
The present study aimed at investigating the efficacy of Tribulus terrestris
on calcium oxalate crystal nucleation and growth in vitro as well as further
examining the potency of Tribulus terrestris on oxalate induced injury
in NRK 52E (rat renal tubular epithelial) cells.
MATERIALS AND
METHODS
Preparation
of the Tribulus terrestris Extract
The
dried and matured fruits of Tribulus terrestris were obtained from “Natural
Remedies Pvt. Ltd.” at Bangalore in India. A collection of voucher
specimens is available at the company.
The air-dried fine powdered plant fruits were boiled in distilled water.
The extract was then filtered using Whatman No. 1 filter paper and the
filtrate was evaporated in vacuum and dried using a rotary evaporator
at 60° C (13). The final dried samples were stored in labeled sterile
bottles and kept at -20° C. The various concentrations of the plant
sample tested for their inhibitory potency were 25 µg/mL, 50 µg/mL,
100 µg/mL, 200 µg/mL, 400 µg/mL and 1000 µg/mL,
which were prepared at the time of experiment and were referred to as
aqueous extract of Tribulus terrestris.
For cell culture studies a stock solution of the dried aqueous Tribulus
terrestris extract was dissolved in dimethyl sulfoxide (DMSO) [final concentration
of the DMSO in the highest concentration of plant extract tested did not
exceed 0.4% (v/v) and did not affect the cell proliferation]. Further
dilutions of the stock were done using serum free DMEM (Dulbecco’s
Modified Eagle’s Media) and filtered by 0.3 mm syringe filter (14).
Nucleation
Assay
The method used was similar to that described by Hennequin et al. with some minor modifications (15). Solutions of calcium chloride and sodium oxalate were prepared at the final concentration of 3 mmoL/L and 0.5 mmoL/L, respectively, in a buffer containing Tris 0.05 moL/L and NaCl 0.15 moL/L at pH 6.5. Both solutions were filtered through a 0.22 µm filter; 33 mL of calcium chloride solution was mixed with 3.3 mL of the aqueous extract at different concentrations. Crystallization was started by adding 33 mL of sodium oxalate solution. The final solution was magnetically stirred at 800 rpm using a PTFE-coated stirring bar. The temperature was maintained at 37o C. The absorbance of the solution was monitored at 620 nm after every 1 min. The percentage inhibition produced by the herb extract was calculated as [1-(Tsi/Tsc)] X 100, where Tsc was the turbidity slope of the control and Tsi the turbidity slope in the presence of the inhibitor.
Growth Assay
Inhibitory activity against CaOx crystal growth was measured using the seeded, solution-depletion assay described previously by Nakagawa and colleagues (16). Briefly, an aqueous solution of 10 mM Tris-HCl containing 90 mM NaCI was adjusted to pH 7.2 with 4 N HC1. Stone slurry (1.5 mg/mL) was prepared in 50 mM sodium acetate buffer (pH 5.7). CaOx monohydrate crystal seed was added to a solution containing 1 mM CaCl2 and 1 mM sodium oxalate (Na2C2O4). The reaction of CaCl2 and Na2C2O4 with crystal seed led to deposition of CaOx (CaC2O4) on the crystal surfaces, thereby decreasing free oxalate that is detectable by spectrophotometry at ?214 nm. When aqueous extract is added into this solution, depletion of free oxalate ions will decrease if the test sample inhibits CaOx crystal growth. Rate of reduction of free oxalate was calculated using the baseline value and the value after 30-second incubation with or without test sample. The relative inhibitory activity was calculated as follows: % Relative inhibitory activity = [(C-S)/C] × 100, where C is the rate of reduction of free oxalate without any test sample and S is the rate of reduction of free oxalate with a test sample.
Cell
Culture
Normal rat epithelial derived renal tubular epithelial (NRK 52E) cells were obtained from National Centre of Cell Sciences (NCCS, Pune). The cells were maintained as monolayers in Dulbecco’s Modified Eagle’s Medium (DMEM) with 2.0 mM L-glutamine adjusted to contain 3.7 g/L sodium bicarbonate, 4.5 g/L glucose. Media was supplemented with 1% Penicillin (100 units/mL)-Streptomycin (10,000 µg/mL) and 10% fetal bovine serum. Cells were cultured in 25 cm2 tissue-culture treated flasks at 37o C and 5% CO2 in humidified chambers.
Oxalate-induced
Cell Injury
NRK 52E cells were incubated in DMEM containing 1 mM sodium oxalate in the presence of different concentrations of the aqueous extract of the test sample (10 µg/mL, 25 µg/mL and 50 µg/mL) for 72 hours (14,17). Cell injury was assessed by measuring the cell viability through trypan blue and monitoring the lactate dehydrogenase (LDH) leakage into the medium.
Cytotoxicity
- Trypan Blue Assay
The cytotoxicity of the aqueous extract of T. terrestris was assessed by cell viability using trypan blue exclusion method. For the determination of cell viability, cells were plated at the density of 4 × 104 cells/well and cultured for 72 h. The medium was replaced with serum-free medium and the cells were treated with various concentrations of the plant extracts (10 µg/mL, 25 µg/mL and 50 µg/mL) for a further 72 h. The percentage viability for the cells was calculated as (live cells/total cells) x 100.
LDH Leakage Assay
LDH leakage assay was performed by the method described by Wagner et al. (18). Briefly, 6.6 mM NADH and 30 mM sodium pyruvate were prepared in Tris (0.2M, pH 7.3). Reaction was initiated with the addition of 50 µL of the test sample and the disappearance of NADH was monitored at 340 nm, for 5 min at an interval of 1 min. The percentage of LDH release was calculated by dividing the activity of LDH in the supernatant by the LDH activity measured after complete cell lysis achieved by sonication.
Statistical
Analysis
Data were expressed as mean values of three independent experiments (each in triplicate) and analyzed by the analysis of variance (p < 0.05) to estimate the differences between values of extracts tested.
RESULTS
Inhibition of Nucleation of CaOx Crystals by Tribulus terrestris Extract
Figure-1 displays the effect of the different concentration of the aqueous extract of Tribulus terrestris on the nucleation of calcium oxalate crystals. As regards control (with no plant sample), the percentage inhibition was constant at 71.4 ± 0.001 with increase in the concentration of Tribulus terrestris extract of 25 µg/mL, 50 µg/mL and 100 µg/mL. As the concentration of Tribulus terrestris extract was increased to 200 µg/mL, the percentage inhibition increased to 100 ± 0.001 but was reduced to 85.7 ± 0.002 for 400 µg/mL. The percentage inhibition was restored to 100 ± 0.001 with 1000 µg/mL of the extract.

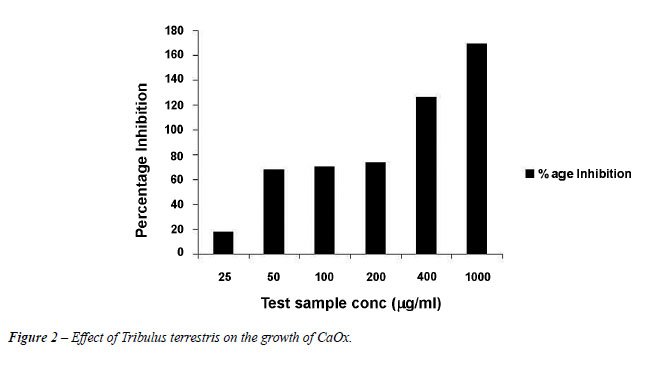
Inhibition
of CaOx Crystal Growth by Tribulus terrestris Extract
Figure-2 demonstrates the percentage inhibition shown by Tribulus terrestris on the calcium oxalate crystal growth. Tribulus terrestris extract showed inhibition in a concentration dependent manner. The percentage inhibition with 25 µg/mL of plant sample was 17.6 ± 0.004. With 50 µg/mL, 100 µg/mL and 200 µg/mL, the inhibition was almost constant in the range of 65-70% but inhibition increased significantly with 400 µg/mL and 1000 µg/mL of Tribulus terrestris extract to 126.4 ± 0.001 and 169.2 ± 0.001 respectively.
Diminution of Oxalate-induced Renal Tubular Epithelial Cell Injury by
Tribulus terrestris Extract
Figure-3
depicts the protective effect of the aqueous extract of Tribulus terrestris
towards the renal tubular epithelial cells. The oxalate induced a significant
injury to the cells which could be ascertained by a decrease in viability
from 100% in the controls (untreated cells) to 73.9%. However, the injury
due to oxalate was significantly reduced in those cells treated with the
Tribulus terrestris extracts. As the concentration of the extract increased
from 10 µg/mL to 50 µg/mL, the percentage viability improved
showing that the plant has an inhibitory activity towards the oxalate
which caused injury to the renal cells in a concentration dependent manner.
The plant extract alone (50 µg/mL, containing 0.4% DMSO) had no
effect on the cell injury in the absence of oxalate indicating that even
at the highest concentration of DMSO used there was no cytotoxicity to
the cells. The percentage viability with 10 µg/mL, 25 µg/mL
and 50 µg/mL was 81.6 ± 6.9, 84.9 ± 1.9 and 89.1 ±
6.9 respectively.
Lactate
dehydrogenase is a stable cytosolic enzyme that is released when the cell
is lysed or there is any injury on the cell membrane. A significant increase
in LDH release was seen when the NRK 52E cells were exposed to oxalate
alone. When NRK 52E cells were treated with the plant extract at varying
concentrations(10, 25 and 50 µg/mL) along with oxalate (1 mM) for
72 h, a reduction in oxalate-induced cell injury was observed as assessed
by a decreased LDH release (Figure-4), Again it was seen that the plant
extract alone had no significant effect on the measures of cell injury
in the absence of oxalate. The percentage LDH release for 10 µg/mL,
25 µg/mL and 50 µg/mL was observed to be 126.5 ± 4.2,
112.6 ± 5.2 and 109.8 ± 1.0 respectively after treatment
with oxalate and the plant extract with respect to control.


COMMENTS
There
is growing evidence that CaOx nephrolithiasis is associated with renal
injury. Hyperoxaluria is a major risk factor for calcium oxalate nephrolithiasis,
and calcium oxalate urinary stones are the most common type of urinary
stone. High level of oxalate produced a variety of changes in the renal
epithelial cells, such as an increase in free radical production and a
decrease in antioxidant status, followed by cell injury and cell death.
These changes are significant predisposing factors for the facilitation
of crystal adherence and retention (5,14).
Due
to significant side effects and failure to prevent recurrence by the present
day treatment procedures for urolithiasis, alternative treatment modalities
using herbal products have assumed importance. A dramatic advancement
in using phytotherapy for urolithiasis treatments has been observed in
recent years and many investigators have proposed to further scientific
study on its efficacy. Many medicinal plants have been employed for centuries
to treat urinary stones though the rationale behind their use is not well
established.
In
the present study, the anticalcifying properties of Tribulus terrestris
commonly called “gokhru” were explored in vitro. The inhibitory
potency of the plant was tested on the nucleation and growth of the most
commonly occurring kidney stones, calcium oxalate monohydrate. A concentration
dependent trend of inhibition was observed using Tribulus terrestris extract
with maximum inhibition of 100% and 170% for CaOx nucleation and the growth
assay respectively with 1000 µg/mL of the extract.
In
our study with NRK 52E, Tribulus terrestris proved to have a protective
effect towards the renal epithelial cells again in a concentration dependent
manner. When NRK-52E cells were injured by exposure to oxalate for 72
h, the plant extract prevented the injury in a dose-dependent manner.
The mechanism of inhibition /reduction in the injury needs to be studied
further. Studies have shown that inhibition of the inflammatory response
induced by injury due to crystal formation helps in restoring normalcy.
Beghalia
et al. (19) have suggested in studies using certain Algerian medicinal
plants that the herb extract may contain substances that inhibit the growth
of COM crystals. This property of plant extracts could be important in
preventing kidney stone formation; the agglomeration of particles is a
critical step in urinary stone formation, as larger crystals are less
likely to pass spontaneously in the urinary tract (8,20). They (19) further
postulated that the plant extracts may contain substances that inhibit
CaOx crystal aggregation and also the binding of the crystals to the renal
epithelial surface. This could explain a decrease in LDH release as seen
in the cells treated with the plant extract compared to those treated
with oxalate alone.
Our
studies are in agreement with the studies previously reported as regards
the anti-urolithiatic potency of Tribulus terrestris on the growth COM
crystals using double diffusion gel growth technique (21). The anti-urolithiatic
ability of the plant is also currently being evaluated in animal models
and has exhibited dose-dependent anti-urolithiatic activity and almost
completely inhibited stone formation further supporting our results (22,23).
Recently several plants including Herniaria hirsuta (24), Phyllanthus
niruri (25) and Bergenia linguata (26) are being explored for their anti-urolithiatic
properties on the basis of their usage in the traditional medicine. Herniaria
hirsuta, a plant from Morocco is also known to exhibit the antilithiatic
activity. The adhesion of the radioactive COM crystals to the Madin Darby
canine kidney cells was studied in the presence and the absence of the
aqueous extract. COM crystal binding to the cells was inhibited by the
extract in a concentration dependent manner (24). In vitro effect of an
aqueous extract of Phyllanthus niruri L., a plant used in Brazilian folk
medicine for the treatment of urolithiasis, on a model of CaOx crystal
endocytosis by Madin-Darby canine kidney cells was investigated by Campos
and Schor. The extract exhibited a potent and effective non-concentration-dependent
inhibitory effect on the CaOx crystal internalization. This response was
present even at very high (pathologic) CaOx concentrations and no Phyllanthus
niruri L.-induced toxic effect could be detected (25). Bergenia ligulata
is a widely used plant in South Asia, mainly India and Pakistan, as a
traditional medicine for treatment of urolithiasis. The crude aqueous-methanolic
extract of Bergenia ligulata rhizome was studied using in vitro and in
vivo methods and the extract showed the anti-urolithic activity through
CaOx crystal inhibition, diuretic, hypermagneseuric and antioxidant effects
(26). Also in our laboratory, antilithiatic potency of Dolichos biflorus
(27) and Trachyspermum ammi (28) has been evaluated in vitro and in vivo.
The most active protein fraction was isolated from these plants and thus
adds a new perspective to study plant fractions for their therapeutic
use as antilithiatic proteins.
CONCLUSION
In conclusion, the aqueous extract of Tribulus terrestris has been shown to possess an ability to inhibit CaOx crystallization in vitro. In addition this extract has also shown cytoprotective properties towards the NRK 52E cells by lowering LDH leakage and increasing the cell viability. Our study suggests the possibility of using Tribulus terrestris as a therapeutic agent to treat urolithiasis and further characterization of its active compound(s) could lead to a new candidate drug for patients with urolithiasis.
ACKNOWLEDGEMENT
The Department of Biotechnology, Government of India, provided funds for this research work.
CONFLICT OF
INTEREST
None declared.
REFERENCES
- Kumar V, Farell G, Deganello S, Lieske JC: Annexin II is present on renal epithelial cells and binds calcium oxalate monohydrate crystals. J Am Soc Nephrol. 2003; 14: 289-97.
- Prasad KVSRG, Sujatha D, Bharathi K: Herbal Drugs in Urolithiasis - A Review. Phycog Rev. 2007; 1: 175-9.
- Verkoelen CF, Romijn JC, de Bruijn WC, Boevé ER, Cao LC, Schröder FH: Association of calcium oxalate monohydrate crystals with MDCK cells. Kidney Int. 1995; 48: 129-38.
- Verkoelen CF, Verhulst A: Proposed mechanisms in renal tubular crystal retention. Kidney Int. 2007; 72: 13-8.
- Khan SR: Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995; 23: 71-9.
- Maroni PD, Koul S, Chandhoke PS, Meacham RB, Koul HK: Oxalate toxicity in cultured mouse inner medullary collecting duct cells. J Urol. 2005; 174: 757-60.
- Jonassen JA, Kohjimoto Y, Scheid CR, Schmidt M: Oxalate toxicity in renal cells. Urol Res. 2005; 33: 329-39.
- Kok DJ, Khan SR: Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994; 46: 847-54.
- Jethi RK, Duggal B, Sahota RS, Gupta M, Sofat IB: Effect of the aqueous extract of an Ayurvedic compound preparation on mineralization & demineralization reactions. Indian J Med Res. 1983; 78: 422-5.
- Barros ME, Schor N, Boim MA: Effects of an aqueous extract from Phyllantus niruri on calcium oxalate crystallization in vitro. Urol Res. 2003; 30: 374-9.
- Kieley S, Dwivedi R, Monga M: Ayurvedic medicine and renal calculi. J Endourol. 2008; 22: 1613-6.
- Miyaoka R, Monga M: Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol. 2009; 35: 396-405.
- Kandil O, Radwan NM, Hassan AB, Amer AM, el-Banna HA, Amer WM: Extracts and fractions of Thymus capitatus exhibit antimicrobial activities. J Ethnopharmacol. 1994; 44: 19-24.
- Moriyama MT, Miyazawa K, Noda K, Oka M, Tanaka M, Suzuki K: Reduction in oxalate-induced renal tubular epithelial cell injury by an extract from Quercus salicina Blume/Quercus stenophylla Makino. Urol Res. 2007; 35: 295-300.
- Hennequin C, Lalanne V, Daudon M, Lacour B, Drueke T: A new approach to studying inhibitors of calcium oxalate crystal growth. Urol Res. 1993; 21: 101-8.
- Nakagawa Y, Abram V, Parks JH, Lau HS, Kawooya JK, Coe FL: Urine glycoprotein crystal growth inhibitors. Evidence for a molecular abnormality in calcium oxalate nephrolithiasis. J Clin Invest. 1985; 76: 1455-62.
- Jeong BC, Kwak C, Cho KS, Kim BS, Hong SK, Kim JI, et al.: Apoptosis induced by oxalate in human renal tubular epithelial HK-2 cells. Urol Res. 2005; 33: 87-92.
- Wagner A, Marc A, Engasser JM, Einsele A: The use of lactate dehydrogenase (LDH) release kinetics for the evaluation of death and growth of mammalian cells in perfusion reactors. Biotechnol Bioeng. 1992; 39: 320-6.
- Beghalia M, Ghalem S, Allali H, Belouatek A, Marouf A: Inhibition of calcium oxalate monohydrate crystal growth using Algerian medicinal plants. J Med Plants Res. 2008; 2: 66-70.
- Wesson JA, Worcester EM, Wiessner JH, Mandel NS, Kleinman JG: Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int. 1998; 53: 952-7.
- Joshi VS, Parekh BB, Joshi MJ, Vaidya AB: Herbal extracts of Tribulus terrestris and Bergenia ligulata inhibit growth of calcium oxalate monohydrate crystals in vitro. J Crystal Growth. 2005; 275: e1403-8.
- Anand R, Patnaik GK, Srivastava S, Kulshreshtha DK, Dhawan BN: Evaluation of antiurolithiatic activity of Tribulus terrestris. Int J Pharmacog. 1994; 32: 217-24.
- Anand R, Patnaik GK, Kulshreshtha DK, Dhawan BN: Activity of certain fractions of Tribulus terrestris fruits against experimentally induced urolithiasis in rats. Indian J Exp Biol. 1994; 32: 548-52.
- Atmani F, Farell G, Lieske JC: Extract from Herniaria hirsuta coats calcium oxalate monohydrate crystals and blocks their adhesion to renal epithelial cells. J Urol. 2004; 172: 1510-4.
- Campos AH, Schor N: Phyllanthus niruri inhibits calcium oxalate endocytosis by renal tubular cells: its role in urolithiasis. Nephron. 1999; 81: 393-7.
- Bashir S, Gilani AH: Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol. 2009; 122: 106-16.
- Bijarnia RK, Kaur T, Singla SK, Tandon C: A novel calcium oxalate crystal growth inhibitory protein from the seeds of Dolichos biflorus (L.). Protein J. 2009; 28: 161-8.
- Kaur T, Bijarnia RK, Singla SK, Tandon C: Purification and characterization of an anticalcifying protein from the seeds of Trachyspermum ammi (L.). Protein Pept Lett. 2009; 16: 173-81.
____________________
Accepted
after revision:
October 29, 2009
_______________________
Correspondence
address:
Dr. C. Tandon
Biotechnology and Bioinformatics
Jaypee University of Information Technology
Waknaghat, 173215, Solan, India
E-mail: tandonchanderdeep@yahoo.com
EDITORIAL
COMMENT
Kidney stone disease is a major health problem in modern societies. As technology evolved, surgical options have gained more acceptance as they provide less invasive approaches, more efficacious results and lesser collateral effects. However, the costs involved are significant and an increasing effort should be continuously made in order to optimize prevention. The article presented by Aggarwal et al. clarifies the efficacy of the herbal Tribulus terrestris on the inhibition of calcium oxalate calculi formation. Herbal medicine has been long used to treat different health conditions including stone disease. However, only more recently efforts began to be made to determine the mechanisms involved and their objective efficacy. In the present evidence-based medicine era this is of utter importance. Herbal medicines may be an alternative to the currently existing medicines providing the additional advantage of minimal or inexistent collateral effects. Other herbal medicines should undergo evaluations in vitro to amplify the urologist’s clinical armamentarium to combat kidney stones.
Dr.
Ricardo Miyaoka
Department of Urologic Surgery
University of Minnesota
Minneapolis, MN, USA
E-mail: miyao002@umn.edu
EDITORIAL COMMENT
In this paper, the authors addressed the potential use
of Tribulus terrestris as the therapeutic agent to treat urolithiasis.
Urolithiasis is characterized by high recurrence rate and among the treatments
used are extracorporeal shock wave lithotripsy and drug treatment, although
there is no satisfactory drug to use in clinical therapy. Thus the prevention
of this disease or its recurrence would be of great interest. Phytotherapy
is a common method used in folk medicine as an alternative for primary
health care in many countries and particularly the potential effect of
many plants to treat urolithiasis has been reported over the past years.
The precipitation of calcium oxalate (CaOx) inside the renal tubules and
the interaction between CaOx crystals and tubular epithelium plays an
important role in the genesis and evolution of urolithiasis, since renal
tubular cells selectively bind and uptake CaOx crystals, a phenomenon
followed by a series of intracellular events that culminate in a cell
damage and death. It was shown that aqueous extract of Tribulus terrestris
was able to inhibit CaOx crystallization in vitro and showed cytoprotective
properties increasing the cell viability.
The extract of plants with antilithiatic properties (Tribulus terrestris,
Phyllanthus niruri, Herniaria hirsute, etc.) has been shown effective
to prevent calculi development in the experimental models in vivo and
in vitro, showing significant effects on many stages of stone formation
including crystallization, aggregation, cellular adherence and adsorption
of macromolecules into the calculi, however, its effects in lithiatic
patients are much less clear. Many reasons can be raised for this difference
such as the treatment onset, number of patients, time of treatment, adhesion
to the treatment, etc. Moreover, it was previously shown (1) that rats
with already formed vesical calculi, the administration of Phyllanthus
niruri had no effect on the calculi size or elimination rate but it induced
a shift in the calculi shape toward a smoother surface and probably more
fragile form, which could contribute to elimination and/or dissolution
of calculi. Overall the available data point to a useful therapeutic application
of these plants, including Tribulus terrestris in lithiatic patients,
mainly as prophylactic agent in those persons who are at high risk to
develop stones since they can potentially interfere with the pathogenesis
of urolithiasis and may represent an attractive alternative for the prevention
of lithiasis of the urinary tract.
REFERENCES
1. Barros ME, Lima R, Mercuri LP, Matos JR, Schor N, Boim MA: Effect of extract of Phyllanthus niruri on crystal deposition in experimental urolithiasis. Urol Res. 2006; 34: 351-7.
Dr. Mirian
A. Boim
Associate Researcher, Renal Division
Federal University of São Paulo
São Paulo, SP, Brazil
E-mail: mirian@nefro.epm.br