REPETITIVE
URETERAL STENTING FOR MANAGEMENT OF TRANSPLANT GRAFT URETERAL OBSTRUCTION
(
Download pdf )
LESTER S. BORDEN JR, VERNON M. PAIS JR, DEAN G. ASSIMOS
Department of Urology, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA
ABSTRACT
Purpose:
To review the use of repetitive stenting in the management of patients
with ureteral obstruction after renal transplantation, with an emphasis
on technique and functional graft outcome.
Materials and Methods: Five adult renal
allograft recipients with ureteral obstruction were managed with repetitive
ureteral stenting. Their hospital records, office notes, and operative
reports were reviewed.
Results: All patients were successfully
managed with retrograde ureteral stenting. They underwent an average of
8.8 stent changes over a mean 34.5 month follow up. No decline in renal
function was observed.
Conclusions: Repetitive stenting is a viable
treatment option for select patients with renal allograft ureteral obstruction.
Key
words: kidney transplantation; ureteral obstruction; catheterization;
stent; graft survival
Int Braz J Urol. 2006; 32: 142-6
INTRODUCTION
The most common urological complication following renal transplantation is ureteral obstruction with a reported incidence of 3-10% (1-3). Percutaneous drainage with or without antegrade ureteral stent placement provides a temporary solution for such problems. Open surgical reconstruction is often the preferred method for correcting this problem. However, there are patients who either fail an open surgical reconstructive or endourologic approaches, or who are deemed not to be good candidates for these procedures. Repetitive ureteral stenting may be a viable option in this setting. Herein, we present our experience with this form of management.
MATERIALS AND METHODS
From
November 1997 to April 2003, 460 renal transplant procedures and 26 combined
pancreas and renal transplant operations were performed at the Wake Forest
University Baptist Medical Center. Five adult male patients were diagnosed
with ureteral obstruction following renal transplantation and were managed
with repetitive ureteral stenting. The mean patient age was 50.4 years
(range 27-67). Ureteral obstruction was diagnosed by new onset hydronephrosis
demonstrated by ultrasonography and increasing serum creatinine levels.
Three patients received deceased donor renal transplants, one received
a living-related transplant, and one received a living-unrelated transplant.
Three had failed attempts at open surgical reconstruction, Boari flap
reconstruction in 1 and uretero-pyelostomy with native ureter in 2, and
declined further attempts at open surgical repair. Two had strictures
longer than 2 cm, were not thought to be good candidates for an endourological
approach and declined open surgical repair. Three patients underwent initial
endoscopic retrograde stent placement and two had initial percutaneous
nephrostomy drainage followed by antegrade stent placement. All subjects
were then managed with retrograde stent changes. It is necessary to explain
why some patients were poor candidates for such repair. Why did not you
try nay endoscopic method like dilation or cold knife incision or laser
incision?
The following approach was employed. Patients
were administered prophylactic antibiotics prior to the induction of general
or regional anesthesia. They were placed in the dorsal lithotomy position
and cystoscopic removal of the indwelling ureteral stent was performed.
The transplant ureteral orifice was identified and cannulated with a 5F
angiographic catheter through which a hydrophilic guide wire was inserted
and then manipulated into the renal collecting system. These maneuvers
were monitored with fluoroscopy. Methylene blue or indigo carmine was
administered intravenously to help identify the ureteral orifice when
it was obscured by surrounding edema. A 5F, 12-14 cm double J stent was
passed over the wire using both endoscopic and fluoroscopic guidance.
The wire was subsequently removed and coiling of both ends of the stent
was confirmed with fluoroscopy. The initial stent change interval was
every 3 months and this was increased if there was no evidence of encrustation
up to a maximum period of 6 months.
RESULTS
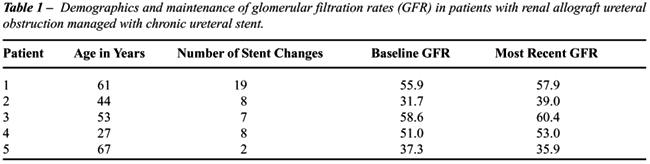
An
average of 8.8 stent changes were performed per patient and the mean duration
of ureteral stenting was 34.5 months (range 9.7-75.5). Which were the
catheter caliber? How much time it remained in the place? Glomerular filtration
rates were calculated by the Cockcroft-Gault formula and measured at baseline
and at the time of the most recent stent change (Table-1). There was no
significant decline in renal function during the period of stenting. There
were no procedure related complications. One patient had periodic, recurrent
urinary tract infections, which were managed with antibiotic therapy.
Which were the symptoms related to double J? It could be interesting to
know about the costs per procedure and per patient/year. I think that
it is elevated.
COMMENTS
Ureteral
complications can develop after both cadaveric and living donor renal
transplantation. They have been reported to occur in up to 10% of patients
undergoing such procedures. Which was the incidence in your casuistic
(1-3)? These complications include urine leak, ureteral necrosis, ureteral
stricture and extrinsic obstruction from lymphocele. The underlying etiology
may be due to surgical technique or compromised vascular integrity of
the ureter. Some of these problems are transient and the patients can
be successfully managed with antegrade or retrograde stent placement,
or nephrostomy tube insertion (4,5). Our cohort included patients who
developed ureteral obstruction who had failed open surgical reconstruction
or had long strictures and declined an open surgical repair. Why not?
While other endourological approaches such as balloon dilation, endoureterotomy
and cutting balloon incision have been used to manage patients developing
ureteral obstruction after renal transplantation, our patient cohort were
not thought to be good candidates for such treatments based on the aforementioned
factors. (6,7). Why? Probably the costs of changing the double J frequently
is more expensive than try a definitive endoscope procedure.
Our results indicate that repetitive stenting
can be a successful form of management for this highly select group; those
who fail open surgical reconstruction, those who are not thought to good
candidates for definitive endourological therapy, and those who decline
open surgical reconstruction. Which kind of patients? In the material
and methods, the patients were not well characterized. Graft function
was maintained at a mean follow-up of almost three years. There were no
complications associated with the stent change procedures. Which were
the criteria for choose the double J caliber? Which was the best caliber?
Which caused fewer symptoms? It was used catheter shorter than the normal
one?
There are great difference between 3 and
6 months. Which the used criterion to make the exchange in 3 or 6 months?
The duration of stenting is a unique feature of our study. Patients were
stented for a mean duration of almost 3 years and a range extending to
75 months. This is the longest reported duration of ureteral stenting
in this population. The only comparable series was reported by Pappas
and associates and the mean duration of stenting was 15 months with a
range of 12 to 21 months (8).
We found that certain technical maneuvers
facilitated stent placement when the ureteral orifice is located in the
dome area of the bladder. Identification of the orifice when bladder inflammation
or bullous edema is present may be aided by the intravenous administration
of indigo carmine or methylene blue. This promotes the excretion of blue
colored urine from the targeted orifice thus facilitating its identification.
Furosemide may also be administered to promote a diuresis if ureteral
efflux is not promptly identified with the latter measures. Alignment
of the scope with the allograft ureteral orifice can be achieved with
any of several maneuvers. The utilization of a 5F angiographic catheter
with a curved tip facilitates guide wire cannulation of the orifice. Manual
suprapubic compression of the bladder may improve the alignment of an
anteriorly located orifice with the angiographic catheter. Finally, flexible
cystoscopy may also allow improved access to the orifice if these prior
maneuvers are unsuccessful. The cannulation itself is aided by the use
of a hydrophilic guidewire. Finally, we strongly recommend that fluoroscopy
be used to monitor these procedures, as the course of the transplanted
ureter may be variable.
CONCLUSIONS
Ideally, definitive correction of transplant graft ureteral obstruction with an open surgical or endourological approach should be considered for most patients with ureteral obstruction after renal transplantation. There is, however, a group of patients who either have failed these approaches or who are not candidates for them. This group was not studied and you cannot conclude on it. Repetitive ureteral stenting is a viable treatment option in this setting and may allow preservation of renal function.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Faenza A, Nardo B, Catena F, Scolari MP, d’Arcangelo GL, Buscaroli A, et al.: Ureteral stenosis after kidney transplantation. A study on 869 consecutive transplants. Transpl Int. 1999; 12: 334-40.
- Shoskes DA, Hanbury D, Cranston D, Morris PJ: Urological complications in 1,000 consecutive renal transplant recipients. J Urol. 1995; 153: 18-21.
- Kinnaert P, Hall M, Janssen F, Vereerstraeten P, Toussaint C, Van Geertruyden J: Ureteral stenosis after kidney transplantation: true incidence and long-term followup after surgical correction. J Urol. 1985; 133: 17-20.
- Sigman DB, Del Pizzo JJ, Sklar GN: Endoscopic retrograde stenting for allograft hydronephrosis. J Endourol. 1999; 13: 21-5.
- Bosma RJ, van Driel MF, van Son WJ, de Ruiter AJ, Mensink HJ: Endourological management of ureteral obstruction after renal transplantation. J Urol. 1996; 156: 1099-100.
- Kristo B, Phelan MW, Gritsch HA, Schulam PG: Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium:YAG laser endoureterotomy. Urology. 2003; 62: 831-4.
- Schwartz BF, Chatham JR, Bretan P, Goharderakhshan R, Stoller ML: Treatment of refractory kidney transplant ureteral strictures using balloon cautery endoureterotomy. Urology. 2001; 58: 536-9.
- Pappas P, Stravodimos KG, Adamakis I, Leonardou P, Zavos G, Constantinides C, et al.: Prolonged ureteral stenting in obstruction after renal transplantation: long-term results. Transplant Proc. 2004; 36: 1398-401.
____________________
Accepted after revision:
January 25, 2006
_______________________
Correspondence address:
Dr. Dean G. Assimos
Department of Urology
Wake Forest Univ. Sch. Med.
Medical Center Boulevard
Winston-Salem, NC 27157, USA
Fax: + 1 336 716-5711
E-mail: dassimos@wfubmc.edu
The
authors describe their interesting experience with patients with ureteral
stenosis after renal transplantation and treated by repetitive ureteral
stenting. Among 460 grafts, 5 cases were included in the program (1.08%).
Three had failed to a previous reimplant surgery and 2 had refused a new
surgical approach.
Ureteral
stenosis occurs in 2 to 10% of renal grafts, being 80% in the uretero-vesical
junction and 71% occurring in the first 3 months after transplantation
(1). Lesions less than 2 cm of extension have better prognosis as well
as those treated during the first 3 months post transplantation (2).
There
are some proposals for the management of ureteral stenosis in graft patients,
but the gold standard approach is the re-anastomosis with the native ureter
(3). However, the minimally invasive or endourological techniques have
been considered to reduce the impact of this complication. Among these
techniques, the balloon dilation, the balloon cutting endoureterotoy (Acucise®),
the Holmium laser endoureterotomy and the prolonged use of double-J stent
are emphasized.
It
is worthy to point out that the retrograde placement of ureteral stent
can be challenging in kidney transplant patients. Nahas et al. described
their experience with retrograde catheterization in patients with renal
graft and ureteral dilation. In 9 of 12 cases (75%), it was possible to
perform successfully retrograde catheterization. The remaining cases were
treated by percutaneous approach (4).
Ureteral
re-implant in kidney transplant patients is object of discussion in medical
literature. Gauleria et al. compared the Leadbetter-Politano ureteral
implant technique with the anterior ureteroneocystostomy and demonstrated
with the second procedure a reduction in the stenosis index (7.7% to 3.8%)
(5).
Kristo
and cols. described the technique of dilation of ureteral stenosis with
a balloon, by anterior approach, in 9 kidney transplant patients. Six
cases were treated exclusively with the balloon and in 3 cases the author
included the Holmium laser for incision. He was successful in all cases,
but all patients presented a stenosis of less than 0.5 cm extension (6).
Balloon
cautery was used by Erturk et al. He reported an 86% resolution index,
but severe bleeding was observed as a complication in one case (7).
The
long-term use of ureteral catheter, described by Pappas et al., is promising.
The stents were replaced each 3 months and remained for a median time
of 15 months. The authors reported 90% of cure of ureteral stenosis in
kidney transplant patients (8).
In
this issue of the International Braz J Urol the authors contribute to
the experience with periodical replacement of ureteral catheter for the
treatment of ureteral stenosis in transplanted patients. In this study,
the use of double-J stent allowed the preservation of renal function in
all cases.
REFERENCES
- Shoskes DA, Hanbury D, Cranston D, Morris PJ: Urological complications in 1,000 consecutive renal transplant recipients. J Urol. 1995; 153: 18-21.
- Goldfischer ER, Gerber GS: Endoscopic management of ureteral strictures. J Urol. 1997; 157: 770-5.
- Schwartz BF, Chatham JR, Bretan P, Goharderakhshan R, Stoller ML: Treatment of refractory kidney transplant ureteral strictures using balloon cautery endoureterotomy. Urology. 2001; 58: 536-9.
- Nahas WC, Gil AO, Mazzucchi E, et al. Retrograde pyelography in transplant kidney: a reliable method. Urol Panamericana. 1995; 7: 39-40.
- Guleria S, Chahal R, Madaan S, Irving HC, Newstead CG, Pollard SG, et al.: Ureteric complications of renal transplantation: the impact of the double J stent and the anterior extravesical ureteroneocystostomy. Transplant Proc. 2005; 37: 1054-6.
- Kristo B, Phelan MW, Gritsch HA, Schulam PG: Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium:YAG laser endoureterotomy. Urology. 2003; 62: 831-4.
- Erturk E, Burzon DT, Waldman D: Treatment of transplant ureteral stenosis with endoureterotomy. J Urol. 1999; 161: 412-4.
- Pappas P, Stravodimos KG, Adamakis I, Leonardou P, Zavos G, Constantinides C, et al.: Prolonged ureteral stenting in obstruction after renal transplantation: long-term results. Transplant Proc. 2004; 36: 1398-401.
___________________________
Dr. Ricardo
Jordão Duarte
Assistant Professor of Urology
University of Sao Paulo, SP, Brazil
E-mail: ricjordao@uol.com.br