CURRENT
APPLICATIONS OF FIBRIN SEALANT IN UROLOGIC SURGERY
(
Download pdf )
L. ANDREW EVANS, ALLEN F. MOREY
Urology Service, Brooke Army Medical Center, Fort Sam Houston, Texas, USA
ABSTRACT
Biosurgical preparations designed to promote surgical hemostasis and tissue adhesion are being increasingly employed across all surgical disciplines. Fibrin sealant is the most widely studied and utilized biosurgical adjunct in urology. Complex reconstructive, oncologic, and laparoscopic genitourinary procedures are those most appropriate for sealant use. This article details the diverse urologic applications of fibrin sealant in the management of genitourinary injuries, surgery, and complications.
Key
words: fibrin sealant; urology; hemostasis; complications; surgery;
biologics
Int Braz J Urol. 2006; 32: 131-41
INTRODUCTION
Although
most applications are off-label, tissue sealants and hemostatic agents
are being increasingly employed across all surgical disciplines. Biosurgical
compounds can serve as adjuncts to primary surgical therapy or may assist
in managing or preventing surgical complications. In urology, hemostatic
agents and tissue sealants are finding increasing roles in managing traumatic
and iatrogenic urologic injuries and promoting optimal wound healing.
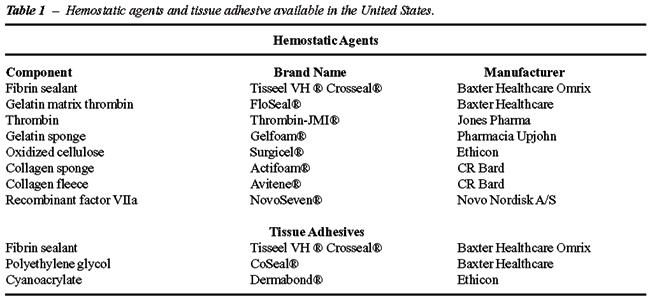
Among the variety of hemostatic products
now available in the United States (Table-1), fibrin sealant is the most
widely utilized biosurgical agent in urologic surgery. This article details
the diverse urologic applications of fibrin sealant for hemostasis, tissue
adhesion, and urinary tract sealing.
FIBRIN
SEALANT
Development
Mixtures of coagulation factors have been
used in surgery for almost a century, dating back to the use of a fibrin
emulsion by Bergel in 1909 to promote wound healing (1). Purified thrombin
became available in 1938, and was first combined with fibrinogen in 1944
to enhance adhesion of skin grafts to burned soldiers (2). Although commercial
fibrin sealant has been widely used in Europe since the 1970’s,
concerns about possible viral transmission limited sealant use in the
United States until recently. In 1998, Tisseel® (Baxter Healthcare,
Deerfield, Illinois) became the first fibrin sealant approved by the Food
and Drug Administration (FDA) for use in the United States.
Although the three FDA approved indications
for fibrin sealant are reoperative cardiac surgery, colon anastomosis,
and treatment of splenic injury, fibrin sealants have been successfully
employed in countless numbers of non-urologic surgical applications, including
liver laceration, hepatic resection, bowel and vascular anastomoses, enterocutaneous
and anorectal fistulae closure, cardiothoracic surgery, and neurosurgery.
A review in 2002 by Shekarriz & Stoller (3) was the first major contemporary
urological publication addressing the use of fibrin sealant in urologic
surgery, and an increasing number of urological sealant applications have
followed.
Composition
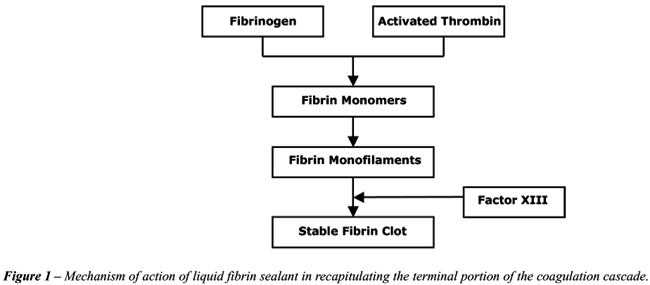
Fibrin sealant contains 2 major components
(thrombin and highly concentrated fibrinogen) which replicate and augment
the final stage of the coagulation cascade—the cleavage of fibrinogen
into fibrin by the action of thrombin—when mixed together. It is
important to note that the fibrinogen concentration of sealant is supraphysiologic,
15 to 25 times higher than that of circulating plasma. The resultant clot
tends to form more rapidly and more reliably than normal. Other key components
of fibrin sealant are Factor XIII, which covalently crosslinks the fibrin
polymer to produce an insoluble fibrin coagulum, and an antifibrinolytic
agent which inhibits fibrinolysis thus preserving the stable fibrin clot
(Figure-1).
Tisseel® (Baxter Healthcare, Deerfield, Illinois)
and Crosseal® (Omrix Biopharmaceuticals, Ltd, Israel) are the two
fibrin sealants currently marketed in the United States. Tisseel®
contains bovine aprotinin as its antifibrinolytic agent. Aprotinin is
a serine protease inhibitor derived from bovine lung that works to limit
fibrinolysis by inhibiting plasmin, kallikrein, and trypsin. Crosseal
utilizes only human-derived proteins by including tranexamic acid as its
antifibrinolytic agent instead of bovine aprotinin. Tranexamic acid is
a synthetic analogue of the amino acid lysine and competes for lysine
binding sites on plasminogen and plasmin, preventing binding to fibrin
and inhibiting fibrinolysis (4).
Safety
All approved fibrin sealant preparations
utilize a combination of donor screening, serum testing and retesting
after 90 days storage, and a two-step vapor heating process to ensure
viral safety (5,6). These steps are highly effective in ensuring viral
safety and, to our knowledge, there are in 2005 still no reported transmissions
of blood-borne viral pathogens associated with the use of FDA approved
fibrin sealants (5). One parvovirus B19 transmission involving a non-FDA
approved fibrin sealant was reported from Japan, but most adults have
preexisting antibodies to this virus and the infection is usually a self-limited
diarrhea (7).
Delivery
Methods
Fibrin sealants are administered using a
dual-chamber delivery system in which one chamber containing fibrinogen
and factor XIII is admixed with the other chamber containing thrombin
directly at the site of application using a “Y” adaptor, allowing
an immediate conversion of fibrinogen to fibrin as the solutions exit
the syringe. Dual lumen catheters ensure smooth, rapid sealant delivery,
and a variety of specialized catheters and cannulae are available for
endoscopic, laparoscopic, and open surgical application. We have also
successfully used a dual lumen peripherally inserted central catheter
(PICC) line for percutaneous transrenal application (8). Polymerization
into the biocompatible fibrin clot is completed within 3 minutes (9),
and the clot is gradually broken down and removed from the site by macrophages
within 2-4 weeks, eventually becoming histopathologically invisible, without
fibrosis or foreign-body reaction (10).
UROLOGICAL APPLICATIONS
Commercial
fibrin sealant is employed for three major reasons in urologic surgery
- as a hemostatic agent, a urinary tract sealant, and/or a tissue adhesive.
A list of the most common urological applications is presented in Table-2.
Fibrin sealant’s unique properties as a hemostatic agent, urinary
tract sealant, and tissue adhesive make it an effective adjunct for managing
complex urologic injury and promoting wound healing in the genitourinary
tract.

Hemostasis
Partial
Nephrectomy
Fibrin sealant has been used since 1979
in open partial nephrectomy (11). The recent advent of minimally invasive
techniques for nephron sparing surgery has resulted in widespread fibrin
sealant use during laparoscopic partial nephrectomy today (12-15). A recent
survey of 193 members of the World Congress of Endourology discovered
68% of surgeons routinely utilized fibrin sealant to assist with hemostasis
during laparoscopic partial nephrectomy (16). Application of fibrin sealant
to the cut surface of the renal parenchymal wound after segmental vascular
and collecting system suture ligation during partial nephrectomy enhances
hemostasis. The fibrin sealant layer can then be supported by a gelatin
or collagen bolster, which is effectively glued into the renal defect
by holding manual pressure on the bolster “sandwich”. In vivo
testing of fibrin sealant in a porcine model of open partial nephrectomy
demonstrated supra-physiological sealing pressures of the renal parenchymal
vasculature (mean 378 mm Hg) and collecting system (mean 166 mm Hg) compared
to unsealed controls (17).
Renal
Trauma
In 1989, Kram and colleagues first reported
fibrin sealant use in 14 patients with traumatic renal injuries: renal
salvage was achieved in all cases with no postoperative infection, delayed
hemorrhage, or urinoma formation (18). In 2004, our laboratory reported
the effective use of FDA-approved fibrin sealant in central porcine renal
stab wounds when used in conjunction with a bolster of absorbable gelatin
sponge or microfibrillar collagen (19). Though not yet commercially available,
the absorbable fibrin adhesive bandage (AFAB), a similar product consisting
of dry fibrin sealant on a polyglactin mesh backing developed in conjunction
with the American Red Cross, significantly reduced bleeding in addition
to operative and ischemic times in repair of porcine models of lower renal
pole amputation (20) and grade IV renal stab wounds (21).
Miscellaneous Hemostatic Applications
Noller et al. reported no hemorrhagic complications
in 10 consecutive renal units treated with fibrin sealant-assisted tubeless
percutaneous nephrolithotomy (PCNL) (22). The instillation of 2 to 3 mL
of fibrin sealant into the parenchymal defect is performed as the sheath
is removed at the conclusion of PCNL in lieu of nephrostomy drainage.
Postoperative computed tomography has confirmed the absence of perirenal
hematomas in these “tubeless” procedures.
We have found that intraoperative splenic
injury during left nephrectomy is easily managed with direct application
of fibrin sealant to the bleeding parenchyma, thereby promoting prompt
hemostasis and avoiding the need for splenectomy (23). Fibrin sealant
has also been successfully used to control “medical” bleeding
caused by warfarin use or other coagulopathies during urologic surgical
procedures (24,25). Other urologic hemostatic applications include sealing
the oral mucosal donor site during buccal graft urethroplasty (26) and
cystoscopic application of fibrin sealant after fulguration to provide
hemostasis in refractory radiation-induced hemorrhagic cystitis after
supravesical urinary diversion (27).
Urinary
Tract Sealant
A variety of non-urological studies has
suggested the increased strength of sealed anastomoses. Skin sutures supported
by a layer of fibrin sealant provided watertight anastomoses immediately
after surgery and withstood significantly higher hydrostatic pressures
than non-sealed anastomoses (28). Han et al. noted that microvascular
sutured anastomoses supported by fibrin sealant had enhanced re-endotheliazation
(29), and Park et al. reported significantly increased tensile strength
in sealed skin closure versus controls (30).
Ureteral
Anastomosis
Kram and colleagues first reported the successful
use of fibrin sealant as a bolster over the suture line for ureteral anastomosis
in 1989 (18). We have found fibrin sealant to be a useful adjunct in managing
a variety of ureteral injuries, both iatrogenic and traumatic, and have
frequently performed “drain-free” sealed repairs. Between
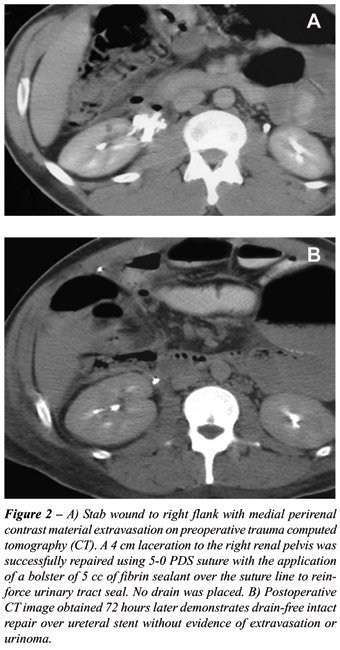
2001 and 2003, 10 patients underwent definitive management of ureteral
injury at our institution. Our experience has shown that sealant effectively
prevents ureteral urinary extravasation and has not been associated with
postoperative infection, leak, or scar formation (Figure-2). We believe
that a sealed, stented ureteral repair is prudent in cases where a transabdominal
approach has been performed because transabdominal drains are avoided.
We also feel it is important to apply the sealant as a means of “suture
support” by reinforcing standard suture lines, not in lieu of careful
suture repair.
The increasing performance of laparoscopic
renal reconstruction surgery may lead to increased sealant use. Fibrin
sealant has been shown to successfully support approximating sutures in
a porcine model of laparoscopic ureteral anastomoses (31) and has improved
radiographic outcomes compared to free needle suturing and laser weld
closure (32). A variety of studies have shown fibrin sealant to be effective
as a bolster for laparoscopic pyeloplasty or collecting system repair
(33), and satisfactory drainage has been confirmed by radiologic imaging
at one to two years (34).
Prostatectomy
Drain-free simple retropubic prostatectomy
has been successfully performed in over 25 cases in our institution, and
we have demonstrated a faster return to regular diet and shortened hospital
stay when compared with conventional simple prostatectomy (35). Again,
we believe it is important to apply the sealant outside the urinary tract,
over the sutured prostatic capsular closure, to ensure that the fibrin
clot does not occlude urinary catheter drainage. Similarly, Diner et al.
reported in 2004 that a significant decrease in postoperative drain output
was noted in 16 patients following radical retropubic prostatectomy when
5 cc of fibrin sealant was applied to the suture line of the urethrovesical
anastomosis (36). Earlier drain removal should facilitate a more expedient
recovery and earlier discharge from the hospital leading to cost savings.
Urethroplasty
Fibrin sealant appears to allow earlier
catheter removal, improved patient satisfaction, and enhanced wound healing
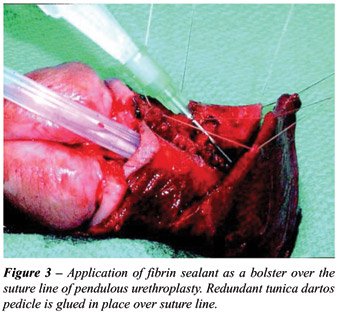
after pendulous urethroplasty (37). In our experience of applying fibrin
sealant directly over a suture line of 5-zero polydiaxanone during pendulous
urethroplasty in 18 patients, a completely healed anastomosis was confirmed
by voiding cystourethrography (VCUG) performed 1 week postoperatively
in 83% of patients; all 18 patients demonstrated complete healing within
14 days, compared to 8% of patients in the control group who had persistent
extravasation at 21 days postoperatively (p < 0.05). Pendulous urethral
reconstruction seems to be uniquely well-suited for sealant use because
the superficial nature of the urethra in this location does not provide
the robust surrounding spongy tissues that are routinely found in the
bulbar urethra (Figure-3).
Complication
Management
Fibrin sealant appears to promote the successful
transvaginal management of iatrogenic cystotomy sustained during transvaginal
hysterectomy. We observed that direct transvaginal fibrin sealant injection
functions well as a bolster interposition over the cystotomy repair, thus
preventing the additional time and morbidity required for abdominal bladder
repair or tissue interposition with a Martius or omental flap (24). Fibrin
sealant has also been utilized to prevent lymphocele formation after lymphadenectomy
(38). Used as a sclerosant after percutaneous drainage of postoperative
lymphoceles in renal transplantation, instillation of fibrin sealant achieved
complete resolution of the lymphocele in 75% of patients without the need
for open surgical management (39). Percutaneous transrenal application
of 5 cc of fibrin sealant across a refractory calyceal urinary leak secondary
to gunshot wound has proven effective in sealing refractory collecting
system injury (8).
Tissue Adhesion
Tissue
Planes
The fibrin polymer resulting from fibrin
sealant application facilitates wound healing by increasing tissue plane
adherence, thus eliminating dead space, accelerating revascularization,
reducing hemorrhage, preventing seroma, and minimizing inflammation (40).
Tissue sealant properties of fibrin sealant
have been applied to reduce air leaks and bronchopleural fistulae after
pulmonary resection and decortication, secure skin grafts in reconstructive
and burn surgery, and occlude chronic enterocutaneous and anorectal fistulous
tracts.
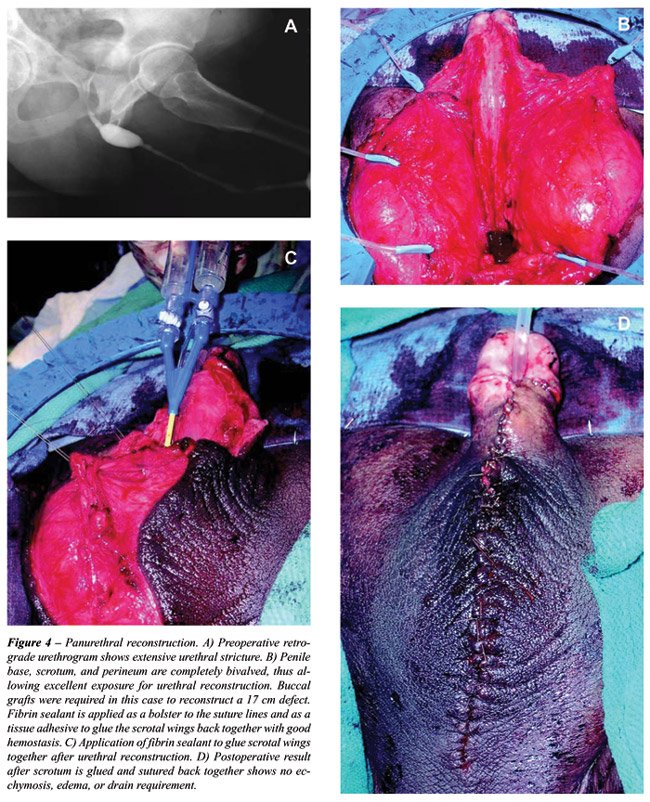
Fibrin sealant is now routinely used at
our institution during complex urethroplasty, especially cases requiring
panurethral reconstruction (Figure-4). The scrotum is completely bivalved
to provide wide access to the underlying diseased urethra, and the scrotal
wings are glued together with sealant after urethral repair to prevent
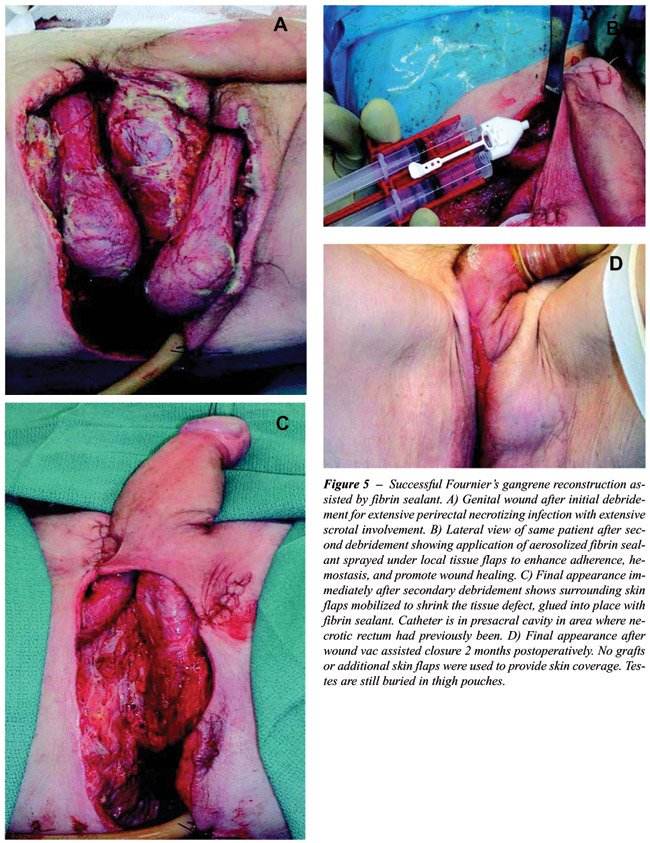
edema and hematoma. Similar efficacy has been reported in 17 patients
undergoing complex genital reconstructive surgery such as spit-thickness
skin grafting and thigh flap surgery for Fournier’s gangrene sequelae
and invasive penile cancer: 94% of patients recovered without infection,
seroma, hematoma, or other complications (Figure-5) (41,42).
Urinary Tract Fistulae
In addition to sealing tissue planes, fibrin
sealant promotes closure of urinary fistulae by promoting the local proliferation
of fibroblasts and subsequent replacement by connective tissue, allowing
for occlusion of the fistulous tract (6). The fibrin polymer promotes
the ingrowth of fibroblasts during wound healing and an influx of immune
cells is stimulated in a paracrine fashion (43). The complex interaction
of neutrophils, macrophages, and fibroblasts provides the basis of wound
contraction and remodeling necessary for healthy wound healing. The recent
application of the Vacuum Assisted Closure® (VAC®, Kinetic Concepts,
Inc., San Antonio, Texas) device in closing larger complex wounds is believed
to function through similar cellular mechanisms (44).
Morita and Tokue reported the successful
closure of a radiation-induced vesicovaginal fistula with the endoscopic
injection of fibrin sealant in combination with bovine collagen (45).
Three serial injections of fibrin sealant allowed for complete continence
in the case of an ureterocutaneous fistula following cadaveric kidney
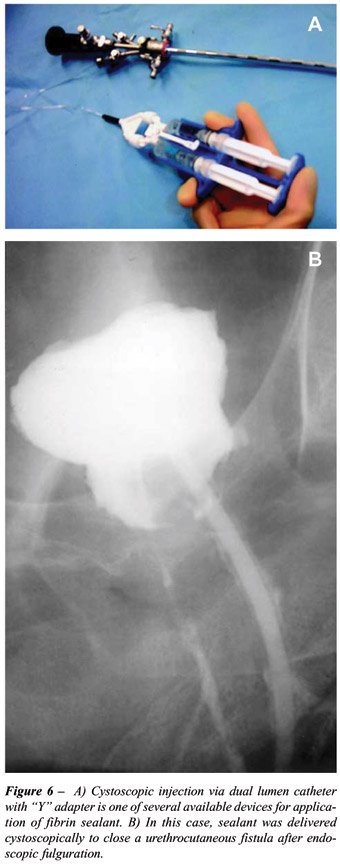
transplantation (46). We reported the successful definitive treatment
of 6 cases of vesicocutaneous and urethrocutaneous fistulae by sealing
the tract with the direct injection of 5 cc commercial fibrin sealant
in conjunction with open or endoscopic fulguration (24) (Figure-6). We
have not found sealant to be effective in vesicovaginal fistula, however,
and this is probably because these fistulas are too short and broad compared
with the long, thin fistulas typically found extending from the lower
urinary tract in males.
SAFETY
CONSIDERATIONS
Fibrin sealant should not be placed into large blood vessels due to the risk for potential thromboembolism. Repeat use of bovine thrombin preparations, which also contain bovine factor V, can induce the formation of antibodies that cross-react with human factor V and lead to a coagulopathic state (47). Pavlovich reported the postoperative development of coagulopathy due to repeat exposure to bovine thrombin during partial nephrectomy (48). The use of bovine-derived proteins carries a risk of allergic reaction upon re-exposure to the material, although bovine aprotinin (found in fibrin sealant) is much less immunogenic than thrombin (49).The reported incidence of hypersensitivity to intravenous aprotinin approaches 10%; thus, fibrin sealant containing bovine protein products should be used with caution in patients previously exposed to aprotinin (50).
CONCLUSIONS
Hemostatic agents and tissue sealants should not be viewed as a replacement for conventional sound surgical judgment or technique, but rather as complementary adjuncts to improve surgical outcome. Fibrin sealant offers an effective adjunct for hemostasis, reinforcement of urinary tract closure, and adhesion of tissue planes. Numerous reports in virtually all surgical disciplines have confirmed the reliable enhancement of wound healing promoted by fibrin sealant. Future development of novel biotherapeutic materials will continue to provide urologists with safe, reliable agents for managing challenging urogenital injuries and complications.
DISCLAIMER
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of Defense or other departments of the U.S. Government.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Bergel S: Uber Wirkungen des Fibrins. Dtschr Med Wochenschr. 1909; 35: 633-65.
- Cronkite EP, Lozner EL, Deaver J: Use of thrombin and fibrinogen in skin grafting. JAMA. 1944; 124: 976-8.
- Shekarriz B, Stoller ML: The use of fibrin sealant in urology. J Urol. 2002; 167: 1218-25.
- Dunn CJ, Goa KL: Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999; 57: 1005-32.
- Jackson MR: Fibrin sealants in surgical practice: An overview. Am J Surg. 2001; 182: S1-S7.
- Spotnitz WD: Commercial fibrin sealants in surgical care. Am J Surg. 2001; 182: 8S-14S.
- Hino M, Ishiko O, Honda KI, Yamane T, Ohta K, Takubo T, et al.: Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br J Haematol. 2000; 108: 194-5.
- Baughman SM, Morey AF, Van Geertruyden PH, Radvany MG, Benson AE, Foley JP: Percutaneous transrenal application of fibrin sealant for refractory urinary leak after gunshot wound. J Urol. 2003; 170: 522-3.
- Sapala JA, Wood MH, Schuhknecht MP: Anastomotic leak prophylaxis using a vapor-heated fibrin sealant: report on 738 gastric bypass patients. Obes Surg. 2004; 14: 35-42.
- Martinowitz U, Saltz R: Fibrin sealant. Curr Opin Hematol. 1996; 3: 395-402.
- Urlesberger H, Rauchenwald K, Henning K: Fibrin adhesives in surgery of the renal parenchyma. Eur Urol. 1979; 5: 260-1.
- Wolf J, Seifman B, Montie J: Nephron sparing surgery for suspected malignancy: Open surgery compared to laparoscopy with selective use of hand assistance. J Urol. 2000; 163: 1659-64.
- Janetschek G, Daffner P, Peschel R, Bartsch G: Laparoscopic nephron sparing surgery for small renal cell carcinoma. J Urol. 1998; 159: 1152-5.
- Pruthi RS, Chun J, Richman M: The use of a fibrin tissue sealant during laparoscopic partial nephrectomy. BJU Int. 2004; 93: 813-7.
- Finley DS, Lee DI, Eichel L, Uribe CA, McDougall EM, Clayman RV: Fibrin glue-oxidized cellulose sandwich for laparoscopic wedge resection of small renal lesions. J Urol. 2005; 173: 1477-81.
- Gerber GS, Stockton BR: Laparoscopic partial nephrectomy. J Endourol. 2005; 19: 21-4.
- Kouba E, Tornehl C, Lavelle J, Wallen E, Pruthi RS: Partial nephrectomy with fibrin glue repair: measurement of vascular and pelvicaliceal hydrodynamic bond integrity in a live and abbatoir porcine model. J Urol. 2004; 172: 326-30.
- Kram HB, Ocampo HP, Yamaguchi MP, Nathan RC, Shoemaker WC: Fibrin glue in renal and ureteral trauma. Urology. 1989; 33: 215-8.
- Griffith BC, Morey AF, Rozanski TA, Harris R, Dalton SR, Torgerson SJ, et al.: Central renal stab wounds: Treatment with augmented fibrin sealant in a porcine model. J Urol. 2004; 171: 445-7.
- Cornum RL, Morey AF, Harris R, Gresham V, Daniels R, Knight RW, et al.: Does the absorbable fibrin adhesive bandage facilitate partial nephrectomy? J Urol. 2000; 164: 864-7.
- Morey AF, Anema JG, Harris R, Gresham V, Daniels R, Knight RW, et al.: Treatment of grade 4 renal stab wounds with absorbale fibrin adhesive bandage in a porcine model. J Urol. 2001; 165: 955-8.
- Noller MW, Baughman SM, Morey AF, Auge BK: Fibrin sealant enables tubeless percutaneous stone surgery. J Urol. 2004; 172: 166-9.
- Canby-Hagino ED, Morey AF, Jatoi I, Perahia B, Bishoff JT: Fibrin sealant treatment of splenic injury during open and laparoscopic left radical nephrectomy. J Urol. 2000; 164: 2004-5.
- Evans LA, Ferguson KH, Foley JP, Rozanski TA, Morey AF: Fibrin sealant for the management of genitourinary injuries, fistulas and surgical complications. J Urol. 2003; 169: 1360-2.
- Martinowitz U, Varon D, Jonas P, Bar-Maor A, Brenner B, Leibovitch I, et al.: Circumcision in hemophilia: the use of fibrin glue for local hemostasis. J Urol. 1992; 148: 855-7.
- Riccabona M: Reconstruction or substitution of the pediatric urethra with buccal mucosa: indications, technical aspects and results. Tech Urol. 1999; 5: 133-8.
- Ouwenga MK, Langston MD, Campbell SC: Use of fibrin sealant in recalcitrant Hemorrhagic cystitis. J Urol. 2004; 172: 1348.
- Oosterlinck W, Cheng H, Hoebeke P, Verbeeck R: Watertight sutures with fibrin glue: an experimental study. Eur Urol. 1993; 23: 481-4.
- Han SK, Kim SW, Kim WK: Microvascular anastomosis with minimal suture and fibrin glue: experimental and clinical study. Microsurgery. 1998; 18: 306-11.
- Park W, Kim WH, Lee CH, Kim DY, Choi JH, Huh JW, et al.: Comparison of two fibrin glues in anastomoses and skin closure. J Vet Med A Physiol Pathol Clin Med. 2002; 49: 385-9.
- McKay TC, Albala DM, Gehrin BE, Castelli M: Laparoscopic ureteral anastomosis using fibrin glue. J Urol. 1994; 152: 1637-40.
- Wolf JS Jr, Soble JJ, Nakada SY, Rayala HJ. Humphrey PA. Clayman RV, et al.: Comparison of fibrin glue, laser weld, and mechanical suturing device for the laparoscopic closure of ureterotomy in a porcine model. J Urol. 1997; 157: 1487-92.
- Patel R, Caruso RP, Taneja S, Stifelman M: Use of fibrin glue and gelfoam to repair collecting system injuries in a porcine model: implications for the technique of laparoscopic partial nephrectomy. J Endourol. 2003; 17: 799-804.
- Eden CG, Sultana SR, Murray KH, Carruthers RK: Extraperitoneal laparoscopic dismembered fibrin-glued pyeloplasty: medium-term results. Br J Urol. 1997; 80: 382-9.
- Morey AF, McDonough RC 3rd, Kizer WS, Foley JP: Drain-free simple retropubic prostatectomy with fibrin sealant. J Urol. 2002; 168: 627-9.
- Diner EK, Patel SV, Kwart AM: Does fibrin sealant decrease immediate urinary leakage following radical retropubic prostatectomy? J Urol. 2005; 173: 1147-9.
- Hick EJ and Morey AF: Initial experience with fibrin sealant in pendulous urethral reconstruction. Is early catheter removal possible? J Urol. 2004; 171: 1547-9.
- Janetschek G, Hobisch A, Hittmair A, Holtl L, Peschel R, Bartsch G: Laparoscopic retroperitoneal lymphadenectomy after chemotherapy for stage IIB nonseminomatous testicular carcinoma. J Urol. 1999; 161: 477-81.
- Chin AI, Ragavendra N, Hilborne L, Gritsch HA: Fibrin sealant sclerotherapy for treatment of lymphoceles following renal transplantation. J Urol. 2003; 170: 380-3.
- Spotnitz WD, Falstrom JK, Rodeheaver GT: The role of sutures and fibrin sealant in wound healing. Surg Clin North Am. 1997; 77: 651-69.
- Decastro BJ, Morey AF: Fibrin sealant for the reconstruction of fournier’s gangrene sequelae. J Urol. 2002; 167: 1774-6.
- Morris MS, Larson RJ, Santucci, RA, Morey AF: Role of fibrin sealant as tissue glue in complex genital reconstructive surgery. J Urol. 2004; 171: 19.
- Gorodetsky R, Vexler A, An J, Mou X, Marx G: Haptotactic and growth stimulatory effects of fibrin(ogen) and thrombin on cultured fibroblasts. J Lab Clin Med. 1998; 131: 269-80.
- Whelan C, Stewart J, Schwartz BF: Mechanics of wound healing and importance of Vacuum Assisted Closure in urology. J Urol. 2005; 173: 1463-70.
- Morita T, Tokue A: Successful endoscopic closure of radiation induced vesicovaginal fistula with fibrin glue and bovine collagen. J Urol. 1999; 162: 1689.
- Tsurusaki T, Sakai H, Nishikido M, Matsuya F, Kanetake H, Saito Y: Occlusion therapy for an intractable transplant-ureteral fistula using fibrin glue. J Urol. 1996; 155: 1698.
- Christie RJ, Carrington L, Alving B: Postoperative bleeding induced by topical bovine thrombin: report of two cases. Surgery. 1997; 121: 708-10.
- Pavlovich CP, Battiwalla M, Rick ME, Walther MM: Antibody induced coagulopathy from bovine thrombin use during partial nephrectomy. J Urol. 2001; 165: 1617.
- Scheule AM, Beierlein W, Lorenz H, Ziemer G: Repeated anaphylactic reactions to aprotinin in fibrin sealant. Gastrointest Endosc. 1998; 48: 83-5.
- MacGillivray TE: Fibrin sealants and glues. J Card Surg. 2003; 18: 480-4.
________
Accepted:
September 30, 2005
_______________________
Correspondence address:
Dr. Allen F. Morey
Chief, Urology Service
Brooke Army Medical Center
3851 Roger Brooke Drive
Fort Sam Houston, TX, 78234, USA
Fax: + 1 210 916 – 5076
E-mail: allen.morey@amedd.army.mil