EFFECT
OF ALLOPURINOL IN CHRONIC NONBACTERIAL PROSTATITIS: A DOUBLE BLIND RANDOMIZED
CLINICAL TRIAL
(
Download pdf )
AMIR M. ZIAEE, HAMED AKHAVIZADEGAN, MOJGAN KARBAKHSH
Labbafinejad Hospital, Urology Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran, and Tehran University of Medical Sciences, Tehran, Iran
ABSTRACT
Introduction:
The exact mechanism of chronic nonbacterial prostatitis has not been yet
elucidated and the outcome with the current management is dismal. In this
trial, we studied the effect of allopurinol in the treatment of this disease.
Materials and Methods: In this randomized
double blind controlled trial, a calculated sample size of 56 were grouped
into “intervention group” who received allopurinol (100 mg
tds for 3 months) with ofloxacin (200 mg tds) for 3 weeks (n = 29) and
“control group” who received placebo tablets with ofloxacin
(n = 27). Patients’ scores based on the National Institute of Health
Chronic Prostatitis Symptom Score were recorded before therapy and then
every month during the study. A four-glass study was performed before
intervention and after 3 months.
Results: The 2 groups were similar regarding
outcome variables. In the first month of study, a significant but similar
improvement in symptom scores was observed in both groups. Microscopic
examination of prostate massage and post-massage samples were also similar
in both groups. No side effects due to allopurinol were observed in patients.
Conclusion: We did not find any advantage
for allopurinol in the management of chronic prostatitis versus placebo
in patients receiving routine antibacterial treatment.
Key
words: allopurinol; chronic nonbacterial prostatitis; urine reflux
Int Braz J Urol. 2006; 32: 181-6
INTRODUCTION
Chronic nonbacterial prostatitis / chronic pelvic pain syndrome (CP/CPPS) is a common reason for urologic visits (1). Despite significant negative impact on patient quality of life (2), the management of the disease has been dismal (3). Because of the heterogeneous nature of this disease, many types of single agents (4) and multimodal therapies (5) have been tried but not proved to be effective. Persson and colleagues hypothesized the role of urate reflux from urine to the prostate in the pathophysiology of the disease for the first time (6) and recommended allopurinol for its treatment in a randomized clinical trial (7). This therapy has not been widely accepted by other urologists because of low response rate reported by others (8). Now in various papers, allopurinol has appeared in the list of potential treatment modalities of chronic prostatitis (9-11). Nevertheless, according to a Cochrane review, provided data are not convincing that allopurinol resulted in the relief of symptoms (12). No other studies have assessed this therapeutic effect. In this study we evaluated the improving effect of allopurinol on clinical signs and symptoms of nonbacterial prostatitis.
MATERIALS AND METHODS
This
was a double blind randomized controlled trial. To calculate the sample
size, we assumed an alpha error of 0.05, a beta error of 0.2 and the mean
scores provided by Persson et al. study (7), the only article similar
to ours. In that trial, the mean symptom score between days 45-135 was
-1.08 (SD = 1.29) for the 25 men in the allopurinol group, compared to
-0.21 (SD = 0.97) for the 14 men in the control group. When the formula
of sample size estimation for comparison of 2 means was applied, it was
established that the sample size had to be 27 patients per group. Thus,
we randomized 56 cases diagnosed with CP/CPPS into 2 groups: intervention
(n = 29) and control (n = 27). The patients were recruited from September
2002 to September 2004. All patients were followed to the end of the study
(no loss to follow-up). According to the prevailing evidence (3,13-15),
the following components were used as the inclusion criteria in this study.
Inclusion criteria - Pain in penis, perineal
region, supra pubic, testis and/or pelvis after ejaculation. Voiding symptoms
such as dysuria, frequency and sense of incomplete urination. Minimum
duration of these symptoms for inclusion in the study was 1 year and minimum
total symptom score 14 (moderate severity of symptom). We included only
those 20 to 40 years old in order to minimize the effect of BPH on symptom
score. A normal abdominal palpation was necessary for inclusion. A classical
4 glass study was performed for each patient which must have been typical
for CP/CPPS for being included (4 negative cultures and inactive at least
for the first 2 specimens) (1).
Exclusion criteria - No past medical history
for documented urinary tract infection (positive urine culture, symptoms
suggesting acute bacterial prostatitis, upper urinary tract infection
and urinary tract tuberculosis), sexually transmitted disease (urethral
discharge, genital ulcer and epididymo-orchitis), urethral stricture (pelvic
fracture, urethral bleeding, urethral instrumentation other than diagnostic
cystoscopy and urethral catheterization), neurological disease (vertebral
column disease, trauma or surgery, disease affecting nervous system such
as multiple sclerosis, cerebrovascular accident), drugs which mimic these
symptoms (for example anticholinergics and psychotropics), urinary system
disease (tumors, stones and interstitial cystitis diagnosed by cystoscopy
or biopsy) and genitourinary system surgery (bladder, kidney, ureter,
vasectomy, hernia, varicocelectomy, etc.).
No evidence of neurological disease (gait
disturbance, abnormal perineal sensation or anal sphincter tone - a mildly
spastic sphincter was considered normal, and spina bifida), genital disease
(ulcer, discharge or scar), prostate nodules.
Regarding paraclinics and imaging, normal
urine analysis and culture were mandatory. Cases with hematuria or pyuria
were excluded from the study. Normal ultrasonography of urinary tract
was another essential para-clinical index (no stones, diverticula, masses,
abnormally thick bladder wall or post-voiding residue above 50 milliliters).
All the included patients were offered information
regarding the explorative nature of the study and consented by written
agreement. They were interviewed before any medical interventions and
then monthly for 3 months using the National Institute of Health (NIH)
prostatitis symptom index (13) translated into Farsi. Translation and
back translation was made by 2 of the authors; one of whom did the translation
and the other who did not know the original English text did the back
translation. The final translation was fixed by consensus of all authors
and was ready to the patients to facilitate communication of symptoms
and improve response rate.
The intervention group received allopurinol
100 mg three-times-daily (tds) for 3 months in addition to ofloxacin for
the 3 first weeks and the control group received placebo tablets (manufactured
exactly similar to the color and shape of allopurinol tablets for the
purpose of this trial) and ofloxacin. The rationale for ofloxacin usage
was being the recommended drug for chronic nonbacterial prostatitis management,
covering culture-negative germs like clamydia (3) and the dosage (200
mg tds instead of 300 mg bid) was chosen to improve compliance (as allopurinol/placebo
were also prescribed tds) (16).
Pain score, urinary symptom score, quality
of life score and total symptom score (the primary major outcome) were
recorded four times for each patient: once before treatment and three
times afterwards in one-month intervals. In the case of patients’
participation, the four-glass test was repeated at the end of the trial.
The patients were also requested a 24-hour urine collection for creatinine
and uric acid before and after the treatment. Age, duration of current
disease, history of alpha-blocker intake and its response, four glass
results and symptom index were recorded for patients.
Scores numerated from baseline through 3
(e.g. total score baseline, total score 1) refer to scores before intervention
(0) and at the corresponding months of drug administration.
In each visit, patients were asked about
any side effects (jaundice, pruritus, rash, and edema).
General Linear Model (repeated measures)
in SPSS 11.5 was used for statistical analysis. P = 0.05 was considered
as the level of statistical significance.
RESULTS
Mean
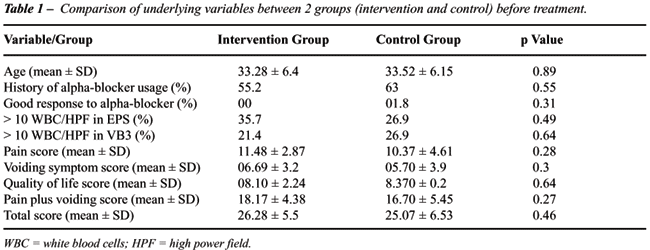
and standard deviation of age was 33.39 ± 6.2. Comparison of underlying
variables between 2 groups before intervention showed no statistical differences
(Table-1).
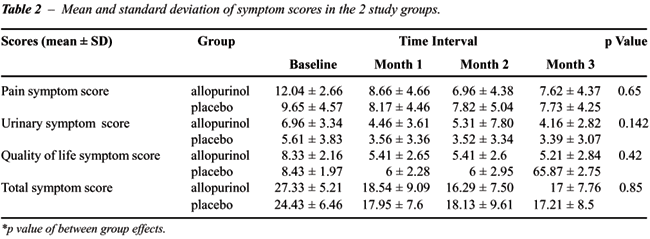
No significant differences between the 2
treatment groups on the study scores were observed (“no between-group
effect”) (Table-2). Nevertheless, significant differences were detected
at the end of the first month “within” each group (Ppain
score = 0.001, Purinary score = 0.05, Pquality of
life score ≤ 0.001 and Ptotal score ≤ 0.001).
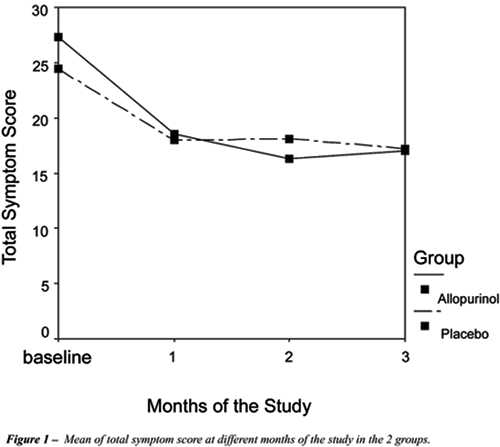
Therefore, the symptom scores decreased nearly 30 percent in the first
month of study in both groups with no significant changes following (Figure-1).
The white blood cells content in 4-glass
test and 24-hour urine collection for uric acid showed no significant
differences, neither within nor between the 2 treatment groups. No side
effects of allopurinol were detected in intervention group.
COMMENTS
Only
one small trial of allopurinol for treatment of chronic prostatitis has
shown improvements in patient-reported symptoms, investigator-graded prostate
pain and biochemical parameters to date (7); but no other evidence exists
to support it (9). In that very research (7), 54 patients (with 39 patients
completing the study) were randomized into 2 groups (placebo and allopurinol)
with significant improvement in the intervention group.
Our study was designed in line with the
CP/CPPS clinical trial reported by the National Institutes of Health Chronic
Prostatitis Collaborative Research Network (13). The NIH/ symptom score
(17), which is a valid questionnaire (18-21) for CP/CPPS, has been used
for scoring the prostatitis symptoms. Persson and colleagues (7), using
their own questionnaire, observed the peak ameliorative effect of allopurinol
after three months. The three-month period for follow-up was decided on
this basis in our trial.
In this study, we did not find any differences
between “allopurinol and ofloxacin” and “placebo and
ofloxacin” in treating CP/CPPS. In the first month of follow up,
symptoms improved significantly in both groups. Nevertheless, no further
improvement was observed in the intervention group in comparison with
the control groups. The improvement of all symptom indices in the first
month might be attributed to initial placebo effect or elimination of
culture-negative germs, with the latter hypothesis being rather farfetched:
in that case, we have to consider “chronic bacterial prostatitis”
as the main etiology of our patients’ symptoms, an otherwise uncommon
condition (22).
Persson’s paper was the only study
reporting the effect of allopurinol on CP/CPPS. In his study, there were
some methodological limitations. Some patients were not in the active
phase of disease, some had positive cultures, some were lost in follow
up, white blood cells in 4 glass test was not measured directly and some
of the symptom scores and P value were not reported (8). Because of these
shortcomings and lack of any other supporting studies, it has been difficult
to verify the effect of allopurinol in chronic nonbacterial prostatitis
(12). In this study we tried to overcome these methodological shortcomings.
Nevertheless, we did not find any preference for allopurinol to placebo
in CP/CPPS management.
Our study has some limitations: first, the
possibility of selection bias: although according to the related literature,
urine analysis and culture, ultra-sonography and four-glass test are considered
enough to confirm the diagnosis of chronic nonbacterial prostatitis (3,14,15),
it is still probable that some patients with other diseases - mimicking
chronic nonbacterial prostatitis symptoms - have been included in our
study (16). Second, the low power of the study due to low number of patients
recruited, according to the calculated sample size. Nevertheless, the
probability that a significant difference really exists is very low considering
the very similar results in the two groups. Third, antibiotic usage in
both groups, which is generally recommended in cases of chronic prostatitis,
may make it difficult to interpret the first-month improvement in patients’
symptoms.
CONCLUSION
Our study showed that allopurinol does not have any ameliorative effect on chronic nonbacterial prostatitis regarding clinical symptoms or improvement of quality of life in comparison with placebo. This disease or syndrome has a collection of symptoms with unknown origins. These symptoms may have diverse etiologies and thus a small subgroup may benefit from allopurinol but we do not recommend the routine use of allopurinol for treatment of CP/CPPS.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Batstone GR, Doble A: Chronic prostatitis. Curr Opin Urol. 2003; 13: 23-9. Review. Erratum in: Curr Opin Urol. 2003; 13: 177. Batstone D [corrected to Batstone G Richard D].
- Turner JA, Ciol MA, Von Korff M, Berger R: Prognosis of patients with new prostatitis/pelvic pain syndrome episodes. J Urol. 2004; 172: 538-41.
- Nickel JC, Downey J, Ardern D, Clark J, Nickel K: Failure of a monotherapy strategy for difficult chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2004; 172: 551-4.
- Krieger JN: The problem with prostatitis. What do we know? What do we need to know? J Urol. 2004; 172: 432-3.
- Shoskes DA, Hakim L, Ghoniem G, Jackson CL: Long-term results of multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2003; 169: 1406-10.
- Persson BE, Ronquist G: Evidence for a mechanistic association between nonbacterial prostatitis and levels of urate and creatinine in expressed prostatic secretion. J Urol. 1996; 155: 958-60.
- Persson BE, Ronquist G, Ekblom M: Ameliorative effect of allopurinol on nonbacterial prostatitis: a parallel double-blind controlled study. J Urol. 1996; 155: 961-4.
- Nickel JC, Siemens DR, Lundie MJ: Re: Ameliorative effect of allopurinol on nonbacterial prostatitis: a parallel double-blind controlled study. J Urol. 1997; 157: 628-9.
- Stevermer JJ, Easley SK: Treatment of prostatitis. Am Fam Physician. 2000; 61: 3015-22, 3025-6. Erratum in: Am Fam Physician 2001; 63: 2129.
- Naber KG, Weidner W: Chronic prostatitis-an infectious disease? J Antimicrob Chemother. 2000; 46: 157-61.
- Doble A: An evidence-based approach to the treatment of prostatitis: is it possible? Curr Urol Rep. 2000; 1: 142-7.
- McNaughton CO, Wilt T: Allopurinol for chronic prostatitis. Cochrane Database Syst Rev. 2002; 4: CD001041.
- Propert KJ, Alexander RB, Nickel JC, Kusek JW, Litwin MS, Landis JR, et al.: Design of a multicenter randomized clinical trial for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002; 59: 870-6.
- Kaplan SA, Volpe MA, Te AE. A Prospective, 1-Year Trial Using Saw Palmetto Versus Finasteride in the Treatment of Category III Prostatitis/Chronic Pelvic Pain Syndrome. J Urol. 2004; 171: 284-8.
- Nickel JC, Downey J, Pontari MA, Shoskes DA, Zeitlin SI: A randomized placebo-controlled multicentre study to evaluate the safety and efficacy of finasteride for male chronic pelvic pain syndrome (category IIIA chronic nonbacterial prostatitis). BJU Int. 2004; 93: 991-5.
- McNaughton Collins M, MacDonald R, Wilt TJ: Diagnosis and treatment of chronic abacterial prostatitis: a systematic review. Ann Intern Med. 2000; 133: 367-81.
- Litwin MS, McNaughton-Collins M, Fowler FJ Jr, Nickel JC, Calhoun EA, Pontari MA, et al.: The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999; 162: 369-75.
- Turner JA, Ciol MA, Von Korff M, Berger R: Validity and responsiveness of the national institutes of health chronic prostatitis symptom index. J Urol. 2003; 169: 580-3.
- Collins MM, O’Leary MP, Calhoun EA, Pontari MA, Adler A, Eremenco S, et al.: The Spanish National Institutes of Health-Chronic Prostatitis Symptom Index: translation and linguistic validation. J Urol. 2001; 166: 1800-3.
- Leskinen MJ, Mehik A, Sarpola A, Tammela TL, Jarvelin MR: The Finnish version of The National Institutes Of Health Chronic Prostatitis Symptom Index correlates well with the visual pain scale: translation and results of a modified linguistic validation study. BJU Int. 2003; 92: 251-6.
- Nickel JC: Prostatitis: lessons from the 20th century. BJU Int. 2000; 85: 179-85.
- Usupbaev AC, Hakimhodjaev ZS, Kim AS. The new diagnostic approaches in assessment of abacterial forms (category III) of the chronic prostatitis. BJU Int September 2002: Vol 90; Suppl 2, pp. 226-7.
____________________
Accepted after revision:
October 10, 2005
_______________________
Correspondence address:
Dr. Amir Mohsen Ziaee
Shahid Dr. Labbafinegad Hospital
9th Boostan Street, Pasdaran Avenue, Tehran, Iran
Fax: + 98 21 254-9088
E-mail: ziaee@hotmail.com