SPERM
BANKING FOR MALE CANCER PATIENTS: SOCIAL AND SEMEN PROFILES
(
Download pdf )
TATIANA C.S. BONETTI, FABIO F. PASQUALOTTO, PRISCILA QUEIROZ, ASSUMPTO IACONELLI JR., EDSON BORGES JR.
Fertility, Assisted Fertilization Center (TCSB, PQ, AIJr, EBJr), Sao Paulo, SP, Brazil and Biotechnology Institute, University of Caxias do Sul (FFP), Caxias do Sul, RS, Brazil
ABSTRACT
Purpose:
Report the characteristics of cryopreserved semen from a cohort of male
cancer patients, attitudes towards cryopreservation and outcomes of semen
samples based on a 12-year cryopreservation program.
Material and Methods: Data from 98 male
cancer patients whose sperm samples were banked were evaluated. Demographic
parameters, semen characteristics, destination of sperm banked samples
and questionnaires answered by the patients regarding cryopreservation
time were evaluated.
Results: The cancer diagnoses were testicle
(56.1%), prostate (15.3%), Hodgkin’s lymphomas (9.2%), non-Hodgkin’s
lymphomas (7.1%), leukemia (3.1%) and other malignancies (9.2%). The patients
with testicular cancer presented lower sperm concentration (p < 0.001);
however, there were no differences with the percentage of normozoospermic
patients among cancer type groups (p = 0.185). A shorter time between
cancer diagnosis and sperm banking was observed for testicular and prostate
cancer patients (p < 0.001). Most of the patients (89.5%) favored sperm
banking as a fertility preservation method.
Conclusions: Although less than 20% of banked
sperm samples were disposed of, the majority of patients related sperm
banking with safe for fertility preservation. Our results show that all
male cancer patients of reproductive age facing cancer treatment could
be offered sperm banking.
Key
words: cancer; fertility; semen; sperm banks
Int Braz J Urol. 2009; 35: 190-8
INTRODUCTION
Cancer
is the leading cause of death in the world, accounting for 7.6 million
mortality cases in 2005. Cancer treatments (surgery, radiotherapy and
chemotherapy) are undertaken to remove malignancies, prolong the patient’s
life and improve their quality of life. Some types of cancer have higher
cure rates than others. When detected early and treated according to best
practices, one-third of cancer cases can be cured (1).
The cure rate of malignancies among patients
with testicular cancer, Hodgkin’s disease, lymphoma, or leukemia
can be as high as 90%. However, depending on the underlying disease, age,
type and dose of therapeutic agent used, and duration of treatment, these
patients might present a post-therapy reproductive dysfunction, with 15-30%
remaining sterile in the long term (2-4). With approximately 15% of male
cancer patients at less than 55 years of age when first diagnosed (5),
the impairment of fertility among surviving young cancer patients who
have not yet started a family has gained increasing clinical importance.
Cytostatic chemotherapy targets cells outside
the G0 phase mainly destroy the rapidly proliferating spermatogonias.
It is often following such treatments that the majority of male cancer
patients develop azoospermia (2). The time for recovery of spermatogenesis
is dose dependent and consequently difficult to predict. It has been reported
that, while male cancer patients receiving low doses of cytostatic agents
may expect recovery of spermatogenesis around 12 weeks after chemotherapy,
permanent azoospermia occur in more than 50% of the patients receiving
high doses (6).
High-dose radiotherapy to the pelvic region
is another important treatment modality in patients with carcinoma in
situ of the testis or cancer of the prostate, rectum and bladder, exposing
patients to high risks of developing permanent infertility. The impairment
of spermatogenesis after radiotherapy is also site- and dose-dependent
(7).
Cancer surgery affecting the genital or
pelvic organs can also have adverse consequences for fertility, namely,
reduced sperm concentration (following unilateral orchiectomy for testicular
cancer) (8), erectile dysfunction (after prostatectomy performed in prostate
cancer patients) (9), or dry ejaculation (from radical retroperitoneal
lymph-node dissections) (10).
Moreover, important alterations of spermatogenesis
can be detected prior to treatment in the majority of young patients with
testicular cancer or lymphoma and are thus unrelated to cytotoxic chemotherapy
(7). Patients should be made aware of the possibility that up to 15% of
male patients will already be azoospermic before they have had any chemotherapy
or radiotherapy treatment (11).
Early reports on sperm banking for oncological
patients showed that few patients had semen samples compatible with successful
cryopreservation employed in intra-uterine insemination (12), and pregnancy
rates remained very poor (13). As a result, many oncologists considered
semen cryopreservation an ineffective, expensive and time consuming fertility
strategy for cancer patients.
However, with the introduction of intracytoplasmic
sperm injection (ICSI), surviving male cancer patients may now have a
better chance of fathering children who are genetically their own, even
with the poorest semen samples (14,15).
Furthermore, recent reports have shown that
DNA fragmentation in sperm samples from oncological patients before undergoing
surgery, chemotherapy or radiotherapy treatments are comparable to those
of infertile male partners in assisted reproduction programs and of men
with proven fertility (16,17).
Currently, the sperm banking and the assisted
reproduction techniques, before and after the treatment respectively,
can be successfully offered to male cancer patients. Ideally, semen cryopreservation
should be performed before cancer treatment is started, and, if possible,
multiples samples should be preserved. The decision to offer each technique
is based on the semen quality pre-freeze and post-thaw. Where adequate
amounts of spermatozoa have been banked and semen quality allows, intra-uterine
insemination using the thawed spermatozoa could be considered, and in
vitro fertilization (IVF) techniques including ICSI are generally recommended
where the quantity of sperm available is small or as deemed necessary
per female pathology (18).
In this study, our specific aims were examining
the pre-freeze semen quality and discussing the social importance of sperm
banking to male cancer patients. In particular, we report the characteristics
of cryopreserved semen from a cohort of male cancer patients, the patients’
attitudes toward semen cryopreservation, and tracking of sperm samples
from a 12-year cryopreservation program.
MATERIALS AND METHODS
Between
July 1996 and January 2008, 98 male cancer patients were referred to our
center for sperm banking before receiving potential gonadotoxic therapy,
chemotherapy, and/or radiotherapy. All patients received complete information
regarding options for future use of sperm samples and the IVF program.
This study was approved by the Institutional
Review Board. Patients gave written informed consent for the study procedures
and the use of their clinical and biological data for research purposes.
Patients were asked to collect ejaculated
semen samples a minimum of three times, except for those patients who
started chemotherapy immediately after enrolment into the sperm cryopreservation
program; those in the latter category collected only one or two samples.
A brief medical history including their diagnosed cancer type was obtained
from all patients and blood samples were screened for infectious diseases.
The initially collected semen samples were
analyzed according to World Health Organization guidelines for concentration
and motility (19) and strictly according to Kruger et al. criteria for
morphology (20).
The semen sample was cryopreserved only
if motile spermatozoa were found regardless of its concentration. Upon
assessment, the semen sample was diluted 1:1 with cryoprotectant (test-yolk
buffer with glycerol). Aliquots of 1 mL were transferred to screw-top
plastic vials and subjected to a slow cooling rate process. Then, the
mixture was frozen at -20ºC for 10 minutes and suspended in vapor
phase nitrogen for 2 hours before being stored in liquid nitrogen until
required.
A 200 µL aliquot was separately cryopreserved
in the same way for post-thaw analysis 24 hours after. To conduct the
post-thaw analysis, samples were thawed at room temperature for 5 minutes,
followed by 37ºC incubation for 5 to 10 minutes. The samples were
washed with culture medium, and the concentration and motility were evaluated
according to WHO guidelines for concentration and motility (19).
Data collected from 98 male cancer patients
whose sperm samples were banked at our center consisted of the following:
(i) Recorded parameters routinely inserted into our center’s data
bank (male age, marriage and parental status, type of cancer and treatment,
period from cancer diagnosis to sample cryopreservation), (ii) The semen
characteristics, (iii) The destinations of sperm banked samples (disposed
of, thawed for our own use, or continuous cryopreservation), and (iv)
Questionnaire responses provided by the volunteers themselves regarding
their worries of fertility preservation and concerns about sperm banking.
Statistical analysis of the data was performed
using the statistical package SPSS v14. The continuous data were expressed
by mean ± standard error of the mean (SE) and compared between
two groups using the Mann-Whitney test, or for multiple groups, analysis
of variance (ANOVA). Categorical data were analyzed using frequencies
and the chi-square estimation. P values < 0.05 were considered statistically
significant.
RESULTS
Ninety-eight
patients were referred for sperm banking before gonadotoxic therapy. The
mean age was 33 years (range: 16-69 years) at the time of cryopreservation.
The higher percentage of patients forming this cohort were diagnosed with
testicular cancer (56.1%, n = 55), followed by prostate cancer (15.3%,
n = 15), Hodgkin’s lymphomas (9.2%, n = 9), non-Hodgkin’s
lymphomas (7.1%, n = 7), leukemia (3.1%, n = 3) and other malignancies
(9.2%, n = 9), which included bladder, stomach, rectum, bone and lung
cancers. All semen samples provided motile spermatozoa and therefore were
suitable for cryopreservation.
Semen characteristics at the time of cryopreservation
for the complete group were as follows: mean sperm concentration (45.4
million/mL, range 0.1-368 million/mL), mean of sperm with progressive
motility (43.8%, range 6-84%), and mean of sperm with normal Kruger’s
morphology (3.5%, range 0-11%). The post-thaw test showed mean of motile
sperm recuperation at 28.5%.
Patient ages and semen features along with
cancer type are shown in Table-1. The patients with prostate cancer were
older than patients of other groups. The semen analysis of patients in
the testicular cancer group presented lower sperm concentration than patients
in other groups, except those with non-Hodgkin’s lymphoma.
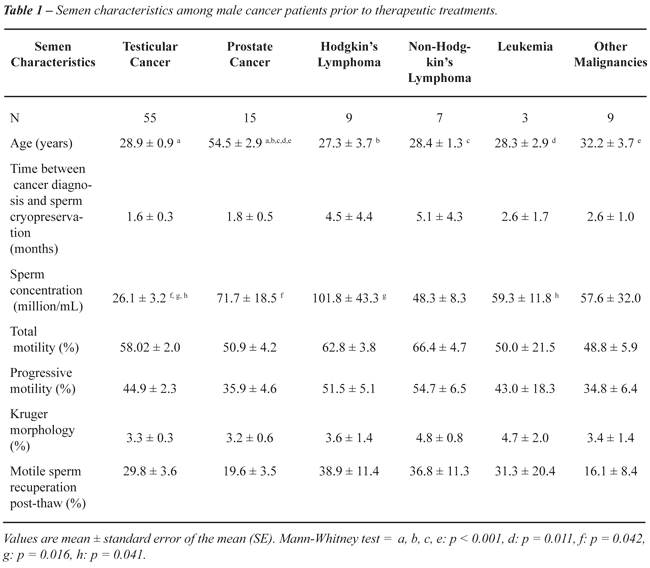
The overall mean time between cancer diagnosis
and sperm cryopreservation was 4.5 months. Although we had not observed
a statistical significance, a shorter time between diagnosis and semen
cryopreservation was observed for patients with testicular and prostate
cancers (Table-1).
Also, the patients were classified according
to sperm concentration as normozoospermia (defined as sperm count ≥
20 million/mL; 59.2%), oligozoospermia (defined as sperm count > 5
million/mL and < 20 million/mL, 20.4%), or severe oligozoospermia (defined
as sperm count ≤ 5 million/mL, 20.4%). However, there was no difference
as regards distribution of study patients grouped by cancer types (p =
0.185).
The social characteristics of patients showed
that only 20.0% of them were married and had children, 28.0% were married
and did not have children, and 52.0% were single and did not have children
at the time of sperm cryopreservation. The prostate cancer group had a
higher percentage of patients who were married (73.3%) and had children
(50%).
After sperm cryopreservation, 39.0% of the
patients received chemotherapy and/or radiotherapy treatment, 35.4% underwent
surgery, and 25.6% had surgery followed by chemotherapy or radiotherapy.
The analysis of responses to the questionnaire
item on cryopreservation time revealed that 78.8% of patients were aware
of their fertility status. The group who expressed being the least concerned
with fertility was the prostate cancer group (46.2%), while 84.7% of the
patients in the other categories expressed awareness of their fertility
status (p = 0.002). This observed difference may be attributed to prostate
cancer patients being older (80% were 55 and older) and who already had
children (50% of prostate cancer group).
Overall, 86.9% of the study patients ranked
fertility as an important issue following cancer treatment. While many
of them already had children, 86.6% of all the study patients still reported
infertility a post-treatment concern. Furthermore, 89.5% of them mentioned
that they felt comfortable with semen cryopreservation regardless of the
type of cancer with which they were diagnosed (p = 0.205).
The sperm samples were cryopreserved for
a mean time of 52.7 months. At the time this report was drafted, 80 samples
(81.6%) remain cryopreserved in our sperm bank. Sperm storage was discontinued
for 18 patients (18.4%) upon the request of either the patient or his
wife. At any time, study patients were able to request their own semen
samples from our center for use in assisted fertilization techniques.
Between 1996 and 1999, 14 cancer patients
agreed to sperm cryopreservation at our center. Since then, an average
of 10.1 cancer patients cryopreserved sperm in our centre per year.
COMMENTS
In
the present study, we retrospectively evaluated the semen characteristics
and attitudes of male cancer patients who had sperm banked before cancer
treatment.
Evidence suggested that cancer patients
have an intrinsic suppression of spermatogenesis due to disease as oligozoospermia
was more frequently observed. The exact mechanism for this suppression
is not well established (21). On the other hand, the patients who suffer
from testicular cancer showed higher semen abnormalities, probably related
to the neoplasm itself (4,11).
Although many studies have reported azoospermia
in cancer patients (11,22), all the patients in the present study provided
motile spermatozoa and therefore were suitable for cryopreservation. Our
findings also demonstrated that the percentage of oligozoospermia in male
cancer patients was high (40.8%) independently of cancer type. However,
an examination of the sperm concentration revealed that it is significantly
lower among testicular cancer patients; thus, this finding supports our
hypothesis that the cancer itself influences spermatogenesis generally
and is amplified in testicular cancer patients.
The decline in semen quality following thawing
is dependent on its initial quality before freezing, but some studies
have demonstrated that the cryopreservation process itself does not affect
spermatozoa of cancer patients any more than that of healthy donors (23).
In this study, we observed a mean post-thaw recuperation rate of 28.5%.
In 2008, it was estimated that there will
be 238,860 new cases of male cancer in Brazil, and 120,330 in the State
of Sao Paulo state (24). Our center serves the State of Sao Paulo. Over
a period of 12 years, only 98 cancer patients cryopreserved semen samples
before cancer treatment. This is, in fact, representative of sperm banking
in Brazil where the number of centers offering sperm banking is small,
and only a limited minority of patients ask for sperm banking.
In our study, testicular cancer patients
more frequently requested sperm cryopreservation, followed by prostate
cancer patients. Also, we found that the mean time from diagnosis of cancer
to the semen collection to cryopreservation was 4.5 months, but this period
for testicular and prostate cancer patients was shorter. Reasons for this
observed difference may be a higher level of awareness of the need for
sperm banking by the medical team treating patients with cancer of the
reproductive organs, or by the patient himself, who then influences the
awareness level of the cancer site regarding fertility issues.
Some authors have raised doubts about the
justification and necessity of providing the facilities for banking spermatozoa
before chemotherapy (25), specially for the reason that the relatively
small number of men making use of it following completion of treatment
is less than 10% (26). The lack of sperm banking that was offered may
be explained by several reasons: recovery or waiting for possible recovery
of gonadal function, short period from original illness, anxiety regarding
potential risks for the children and uncertainty about their long-term
health and therefore their suitability to be parents (18).
In addition, a lack of discussion time,
presumed high cost, unavailability of adequate facilities and overestimation
of the limitations of sperm quality were the most reported reasons why
sperm banking was not suggested (27).
At our center, the cost of sperm banking,
including three samples of semen, is approximately U$ 500.00, with additional
U$ 140.00 per semester for maintaining the cryopreserved sample; and for
Brazilian patients, these costs are high.
Cancer patients can lose interest in preserving
fertility when they are faced with an unpredictable and unfavorable prognosis.
The collection of ejaculate is often difficult due to poor general health
condition. The oncologist may also take a pessimistic view of survival
rate for patients with aggressive cancer, thus diminishing the likelihood
of sperm banking (28).
The most common reason for failing to bank
sperm is a lack of awareness that such an option exists. Instead, many
patients are left with significant anxieties over reproductive health
concerns (28). On the other hand, assuring a patient that his fertility
potential is secured by sperm banking could help in the emotional battle
against cancer (29).
In this study, we found that most of the
study patients were aware of fertility issues, that they expressed post-treatment
infertility as an important concern, and that they were comforted by sperm
banking as a means of fertility preservation.
Increasing awareness and the use of assisted
reproductive technologies need to be promoted by an interdisciplinary
team of experts caring for adolescents and young adults, as sperm cryopreservation
is an efficacious method for preserving future fertility (30). All male
cancer patients of reproductive age who will have treatment that may affect
testicular function should have their sperm cryopreserved before the initiation
of therapy.
Among the study cohort, less than 20% of
banked sperm samples were disposed of upon the request of either the patients
or their spouses. Reasons for disposal of sperm sample were patient death,
no plans for more children, and recovery of fertility. However, at our
center, we do not actively follow-up patients after completion of their
cancer treatment; therefore, we do not have data on their survival or
whether they have been able to conceive spontaneously.
While fertility preservation for post-pubertal
male cancer patients has been well established (with sperm banking and
techniques of assisted reproduction), there is little agreement regarding
appropriate indications for and methods of gamete preservation in pre-pubertal
boys (31). For pre-pubertal boys, the prevention of sterility in childhood
cancer survivors given the existing practices is a clinical challenge
since no active spermatogenesis is yet present. A promising advancement
that has been proposed in the scientific community is cryobanking of testicular
tissue as an acceptable strategy (32). However, this proposal faces a
wall of ethical research debates regarding the conduct of experimentation
on pre-pubertal individuals.
Comprehensive cancer treatment planning
is needed to help oncologists offer sperm banking as an option to all
men at risk of infertility, due to cancer itself or its treatment. The
improvement in cancer treatment and life expectancy, combined with greater
awareness for fertility options, careful reassurance of the survivors
regarding the safety of their children, the possibility of infertility
treatment by assisted reproductive technology, and the beneficial contribution
in the emotional battle against cancer all lend support to routinely offering
sperm banking to all cancer patients, especially those who are interested
in having children with their partners.
CONFLICT OF INTEREST
None declared.
REFERENCES
- WHO. Cancer. 2006 [cited 2007 24/08/2007]; Fact Sheet N°297 World Health Organization. www.who.int/mediacentre/factsheets/fs297/en/print.html.
- Schrader M, Müller M, Straub B, Miller K: The impact of chemotherapy on male fertility: a survey of the biologic basis and clinical aspects. Reprod Toxicol. 2001; 15: 611-7.
- Naysmith TE, Blake DA, Harvey VJ, Johnson NP: Do men undergoing sterilizing cancer treatments have a fertile future? Hum Reprod. 1998; 13: 3250-5.
- Meirow D, Schenker JG: Cancer and male infertility. Hum Reprod. 1995; 10: 2017-22.
- Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW, Lacour B, et al.: Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004; 364: 2097-105.
- Pont J, Albrecht W: Fertility after chemotherapy for testicular germ cell cancer. Fertil Steril. 1997; 68: 1-5.
- Magelssen H, Brydøy M, Fosså SD: The effects of cancer and cancer treatments on male reproductive function. Nat Clin Pract Urol. 2006; 3: 312-22.
- Petersen PM, Skakkebaek NE, Rørth M, Giwercman A: Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol. 1999; 161: 822-6.
- Montorsi F, Briganti A, Salonia A, Rigatti P, Burnett AL: Current and future strategies for preventing and managing erectile dysfunction following radical prostatectomy. Eur Urol. 2004; 45: 123-33.
- Klein EA: Open technique for nerve-sparing retroperitoneal lymphadenectomy. Urology. 2000; 55: 132-5.
- Lass A, Akagbosu F, Abusheikha N, Hassouneh M, Blayney M, Avery S, et al.: A programme of semen cryopreservation for patients with malignant disease in a tertiary infertility centre: lessons from 8 years’ experience. Hum Reprod. 1998; 13: 3256-61.
- Waxman J: Cancer, chemotherapy, and fertility. Br Med J (Clin Res Ed). 1985; 290: 1096-7.
- Scammell GE, White N, Stedronska J, Hendry WF, Edmonds DK, Jeffcoate SL: Cryopreservation of semen in men with testicular tumour or Hodgkin’s disease: results of artificial insemination of their partners. Lancet. 1985; 2: 31-2.
- Horne G, Atkinson A, Brison DR, Radford J, Yin JA, Edi-Osagie EC, et al.: Achieving pregnancy against the odds: successful implantation of frozen-thawed embryos generated by ICSI using spermatozoa banked prior to chemo/radiotherapy for Hodgkin’s disease and acute leukaemia. Hum Reprod. 2001; 16: 107-9.
- Ginsburg ES, Yanushpolsky EH, Jackson KV: In vitro fertilization for cancer patients and survivors. Fertil Steril. 2001; 75: 705-10.
- Ribeiro TM, Bertolla RP, Spaine DM, Fraietta R, Ortiz V, Cedenho AP: Sperm nuclear apoptotic DNA fragmentation in men with testicular cancer. Fertil Steril. 2008; 90: 1782-6.
- Meseguer M, Santiso R, Garrido N, Fernandez JL: The effect of cancer on sperm DNA fragmentation as measured by the sperm chromatin dispersion test. Fertil Steril. 2008; 90: 225-7.
- Lass A, Akagbosu F, Brinsden P: Sperm banking and assisted reproduction treatment for couples following cancer treatment of the male partner. Hum Reprod Update. 2001; 7: 370-7.
- WHO. Laboratory Manual for the Examination of Human Semen and Semen Cervical Mucus Interaction. 1999, 4th ed., Cambridge University Press.
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Veeck LL, et al.: New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987; 30: 248-51.
- Trottmann M, Becker AJ, Stadler T, Straub J, Soljanik I, Schlenker B, et al.: Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol. 2007; 52: 355-67.
- Tal R, Botchan A, Hauser R, Yogev L, Paz G, Yavetz H: Follow-up of sperm concentration and motility in patients with lymphoma. Hum Reprod. 2000; 15: 1985-8.
- Agarwal A: Semen banking in patients with cancer: 20-year experience. Int J Androl. 2000; 23(Suppl 2): 16-9.
- INCA/MS, Cancer in Brazil. Data from Register of Populacional Basis. Cancer National Institute, Ministry of Healthy. 2003. http://www.inca.gov.br/regpop/2003. [in Portuguese]
- Radford J, Shalet S, Lieberman B: Fertility after treatment for cancer. Questions remain over ways of preserving ovarian and testicular tissue. BMJ. 1999; 319: 935-6.
- Lass A: The need for more CEPHS. Fertil Steril. 2000; 73: 418.
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al.: American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006; 24: 2917-31. Erratum in: J Clin Oncol. 2006; 24: 5790.
- Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S: Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002; 20: 1880-9.
- Saito K, Suzuki K, Iwasaki A, Yumura Y, Kubota Y: Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer. 2005; 104: 521-4.
- Neal MS, Nagel K, Duckworth J, Bissessar H, Fischer MA, Portwine C, et al.: Effectiveness of sperm banking in adolescents and young adults with cancer: a regional experience. Cancer. 2007; 110: 1125-9.
- Glaser A, Wilkey O, Greenberg M: Sperm and ova conservation: existing standards of practice in North America. Med Pediatr Oncol. 2000; 35: 114-8.
- Tournaye H, Goossens E, Verheyen G, Frederickx V, De Block G, Devroey P, et al.: Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum Reprod Update. 2004; 10: 525-32.
____________________
Accepted after revision:
December 11, 2008
_______________________
Correspondence address:
Dr. Edson Borges Jr.
Fertility, Centro de Fertilização Assistida
Av. Brigadeiro Luís Antônio, 4545
São Paulo, 01318-000, SP, Brazil
E-mail: edson@fertility.com.br
EDITORIAL COMMENT
The
article by Bonetti and colleagues highlights the importance of understanding
and awareness of the benefits of sperm cryopreservation in a multi-disciplinary
team of health care professionals. Our experience in Canada from a retrospective
chart review (1) illuminated the fact that we needed to address the multi-disciplinary
team to negate the gap in complimentary service between health care professionals
in both cancer and fertility specialties.
Our research partnership aimed at increasing
the use of fertility preservation strategies. Following an extensive literature
review we formed our framework. The need for heightened awareness of the
opportunities for patients to preserve their fertility then became our
focus. It was quickly determined that we needed to have a two-pronged
approach to solving this dearth of information. One would focus on the
allied health care professionals while the other would focus on the patient.
The first step in this process focused on
empowering staff to ensure referrals were made to the Fertility Clinic.
An algorithm for identifying patients was developed to aid in the identification
of candidates for fertility preservation sooner rather than later in treatment.
This afforded the patient the appropriate time to consider his fertility
preservation option(s) and still have the time to bank samples prior to
treatment. In addition, creating a standard referral approach facilitated
staffs’ discussions and eased their discomfort about discussing
sperm banking, especially among younger patients. Increased awareness
and more rigorous clinical approach including the use of the Referral
Form resulted in a 71% increase in sperm cryopreservation referrals compared
to the previous year.
A further project investigated Nurses’
perception(s) of discussions with patients regarding the patients’
future fertility (2). An underlying purpose of this study was to determine
any communication barriers and to ascertain what type of educational materials
would be beneficial.
Since the use of sperm banking, as part
of the treatment protocol for adolescent and young adult (AYA) males with
cancer, requires the expertise and cooperation of a multidisciplinary
team of oncology and fertility experts nurses’ have a primary contact
with patients, their role in effective communication information to patients
is crucial. Therefore, patients’ awareness and understanding of
sperm banking is a key element to success. Patients need to make informed
decisions at a time when they are inundated with treatment information.
A parallel consideration is the fact that
the ability for a cancer survivor to one-day has their own family is of
paramount importance to their quality of life during and after treatment.
With this in mind, “plain language” education materials were
developed to adequately inform male AYA oncology patients about sperm
banking prior to cancer treatment (3). The project involved a collaborative
partnership among health professionals from both the cancer and fertility
clinics.
The educational brochures are beneficial
for initiating discussion with the patient. Using the educational brochure
as a teaching tool has led to the development of expertise and high comfort
level among staff and expertise in facilitating these sensitive discussions.
The key points in this process are listed below, and have become part
of the routine standard of care: 1) Ensure that the health care provider
gives all the appropriate information to the patient so that the patient
can make an informed decision and be successful in providing an ejaculate
for banking, 2) Ease the discomfort that is often felt by health care
providers when discussing sperm banking, 3) Provide a timely referral
to the fertility clinic.
Adolescents and young adults with cancer
are a unique group. Due to many external factors and changes that take
place during this time in their lives the diagnosis of cancer can be overwhelming.
Providing AYAs with evidence-based information about fertility preservation
by staff trained to impart this information allows them to make informed
decisions about their fertility preservation options. Our framework for
coordinating efforts in providing fertility preservation options to patients
undergoing treatment for cancer encourages the use of effective multi-disciplinary
teams that include: oncologists; nurses in both specialties of oncology
and infertility, social work, reproductive endocrinology and infertility
specialists, andrologists, and embryologists are required to work together
in order to achieve success. The result of this unique team approach is
not only a cancer survivor but one that is able to round out their quality
of life by being able to have a family of their own.
REFERENCES
- Neal MS, Nagel K, Duckworth J, Bissessar H, Fischer MA, Portwine C, et al.: Effectiveness of sperm banking in adolescents and young adults with cancer: a regional experience. Cancer. 2007; 110: 1125-9.
- Nagel K, Neal M: Discussions regarding sperm banking with adolescent and young adult males who have cancer. J Pediatr Oncol Nurs. 2008; 25: 102-6.
- Nagel K, Wizowski L, Duckworth J, Cassano J, Hahn SA, Neal M: Using plain language skills to create an educational brochure about sperm banking for adolescent and young adult males with cancer. J Pediatr Oncol Nurs. 2008; 25: 220-6.
Dr.
Michael S. Neal
Centre for Reproductive Care
Hamilton Health Sciences
Hamilton, Ontario, Canada
Fax: 905 521-2100 x 76515
E-mail: nealm@hhsc.ca
Dr.
Kim Nagel
McMaster Child Health Research Institute
McMaster University
Hamilton, Ontario, Canada