T2-WEIGHTED
ENDORECTAL MAGNETIC RESONANCE IMAGING OF PROSTATE CANCER AFTER EXTERNAL
BEAM RADIATION THERAPY
(
Download pdf )
ANTONIO C. WESTPHALEN, JOHN KURHANEWICZ, RUI M. G. CUNHA, I-CHOW HSU, JOHN KORNAK, SHOUJUN ZHAO, FERGUS V. COAKLEY
Department of Radiology, Abdominal Imaging Section, University of California San Francisco, San Francisco, California, USA
ABSTRACT
Purpose:
To retrospectively determine the accuracy of T2-weighted endorectal MR
imaging in the detection of prostate cancer after external beam radiation
therapy and to investigate the relationship between imaging accuracy and
time since therapy.
Materials and Methods: Institutional review
board approval was obtained and the study was HIPPA compliant. We identified
59 patients who underwent 1.5 Tesla endorectal MR imaging of the prostate
between 1999 and 2006 after definitive external beam radiation therapy
for biopsy-proven prostate cancer. Two readers recorded the presence or
absence of tumor on T2-weighted images. Logistic regression and Fisher’s
exact tests for 2x2 tables were used to determine the accuracy of imaging
and investigate if accuracy differed between those imaged within 3 years
of therapy (n = 25) and those imaged more than 3 years after therapy (n
= 34). Transrectal biopsy was used as the standard of reference for the
presence or absence of recurrent cancer.
Results: Thirty-four of 59 patients (58%)
had recurrent prostate cancer detected on biopsy. The overall accuracy
of T2-weighted MR imaging in the detection cancer after external beam
radiation therapy was 63% (37/59) for reader 1 and 71% for reader 2 (42/59).
For both readers, logistic regression showed no difference in accuracy
between those imaged within 3 years of therapy and those imaged more than
3 years after therapy (p = 0.86 for reader 1 and 0.44 for reader 2).
Conclusion: T2-weighted endorectal MR imaging
has low accuracy in the detection of prostate cancer after external beam
radiation therapy, irrespective of the time since therapy.
Key
words: prostate cancer; radiotherapy; follow-up studies; magnetic
resonance imaging
Int Braz J Urol. 2009; 35: 171-82
INTRODUCTION
Approximately 30% of patients with newly diagnosed prostate cancer undergo external beam radiation therapy (EBRT) as their initial definitive treatment (1). Up to 50% of these patients develop biochemical failure (rising serum prostatic-specific antigen [PSA] after a nadir level has been reached) within 5 years, depending on pre-treatment risk factors (2,3). Biochemical failure may be due to local or systemic recurrence or both (3). Irrespective of the PSA trend, identification of tumor in the treated gland early after completion of radiation therapy is important, because the presence of tumor at needle biopsy performed 2-3 years after radiation, even in patients without clinical or biochemical recurrence, is an important predictor of long-term outcome (4,5). However, a non-invasive alternative to transrectal biopsy would clearly be preferable for post-radiation monitoring. Over the last decade, MR imaging has emerged as a powerful tool for locoregional evaluation of prostate cancer. The use of MR imaging after radiation therapy is controversial because post-radiation changes such as prostatic atrophy, the development of diffuse low T2 signal intensity, and indistinctness of the normal zonal anatomy might adversely impact the accuracy of T2-weighted MR imaging (6-8). To our knowledge, only five other studies that in total enrolled just 146 patients have previously investigated the method in this same setting, with inconsistent results that range from low to moderate accuracy (9-13). The existing literature has not systematically reported the influence of time since therapy on the accuracy of MR imaging, although there are good reasons to believe this might be an important variable. For example, it is likely that post-radiation MR changes are at least in part reversible. Pickett et al. showed that 26 months or more after EBRT, 60% of patients present with areas of the prostate that have normal metabolism on serial MR spectroscopic imaging (14). It is conceivable that the diverging results reported by prior studies are influenced by the time interval since radiation. Therefore, we undertook this study to retrospectively determine the accuracy of T2-weighted endorectal MR imaging in the detection of prostate cancer after external beam radiation therapy, and to investigate the relationship between imaging accuracy and time since therapy.
MATERIAL AND METHODS
Patients
This was a retrospective single institution
study approved by our Committee on Human Research with waiver of informed
consent. The study was compliant with requirements of the Health Insurance
Portability and Accountability Act. We retrospectively identified, through
a cross-correlated computerized search of our medical and radiology information
systems, all patients who met the following inclusion criteria:
1
- Definitive treatment of biopsy-proven prostate cancer with external
beam radiation therapy with or without associated neoadjuvant/adjuvant
androgen deprivation therapy.
2 - Post-treatment 1.5 Tesla endorectal
MR imaging of the prostate performed between January 1999 and December
2006.
3 - Post-treatment transrectal ultrasound-guided
biopsy of the prostate performed within 180 days of MR imaging.
4 - No additional treatment for prostate
cancer.
Fifty-nine
patients fulfilled these criteria. The information was redacted for bind
review. Eleven of these men were included in a prior preliminary study
investigating the use of MR imaging and MR spectroscopic imaging for detection
of tumor after radiation therapy (9).
The study group consisted of 59 men with
a mean age of 68.8 years (range, 45.2 to 81.6), a mean pretreatment serum
PSA level of 18.2 ng/mL (range, 3.5 to 93.0), and the following pretreatment
clinical stage (American Joint Committee on Cancer) established on digital
rectal examination: T1 (n = 9/59, 15.3%), T2 (n = 31/59, 52.5%), T3 (n
= 14/59, 23.7%), or unknown (n = 5/59, 8.5%). The median Gleason score
was 7 (range, 5 to 9). The D’Amico risk stratification was based
on the clinical stage, PSA level, and Gleason score (15). Patients were
categorized as having low risk (n = 7/59, 11.9%), intermediate risk (n
= 26/59, 44.1%), or high risk (n = 26/59, 44.1%) tumor.
Forty-two patients received a mean dose
of 74.6 Gy (range, 65-82 Gy); the dose administered to 17 patients treated
at outside institutions was unknown, but all completed a full course of
standard radiotherapy. Seventeen patients (17/59, 28.9%), 5 (5/59, 8.5%),
and 6 (6/59, 10.2%) patients underwent neoadjuvant, adjuvant, or neoadjuvant
plus adjuvant hormonal therapy for a mean duration of 3.9 months (range,
2 to 5), 8.6 months (range, 5 to 12), and 13.3 months (range, 4 to 21),
respectively.
The mean interval from external beam radiation
therapy to MR imaging was 44 months (range, 17-138 months), The mean interval
between MR imaging and biopsy was 60 days (range, 0-175 days) and most
procedures were performed within 90 days of imaging (78%, 46/59).
Patients underwent MR imaging to assess
suspected local recurrence on the basis of rising PSA. At the time of
imaging, twenty-two patients (22/59, 37.3%) had biochemical failure, defined
as nadir + 2 ng/mL (16). All patients were biochemically disease free
following EBRT.
MR
Imaging Technique
Patients were scanned in a supine position
using the body coil for excitation and a pelvic phased array coil (GE
Medical Systems, Milwaukee, WI) in combination with a balloon-covered
expandable endorectal coil (Medrad, Pittsburgh, PA) for signal reception
on a 1.5-Tesla whole body MR scanner (Signa; GE Medical Systems, Milwaukee,
WI). The following parameters were used for acquisition of T1-weighted
spin-echo MR images of the pelvis: TR/TE 766/8, slice thickness = 5 mm,
interslice gap = 1.5 mm, field of view = 24 cm, matrix 256 x 192, anteroposterior
frequency encoding, and 1 excitation. Thin-section high nominal spatial
resolution axial and coronal T2-weighted fast spin-echo images of the
prostate and seminal vesicles were acquired with the following parameters:
TR/effective TE 5000/96 ms, echo train length = 16, slice thickness =
3 mm, interslice gap = 0 mm, field of view = 14 cm, matrix 256 x 192,
anteroposterior frequency encoding (to prevent obscuration of the prostate
by endorectal coil motion artifact), and 3 excitations.
Imaging
Interpretation
Two radiologists, with experience in genitourinary
radiology, independently reviewed all the images. The radiologists knew
patients were treated with external beam radiation therapy for prostate
cancer and that all patients had rising PSA values, but had no access
to any other clinical or histological information. Images were reviewed
at a picture archiving and communication system workstation (Impax; Agfa,
Mortsel, Belgium). The following MR imaging data was recorded:
• Presence or absence of post-biopsy
hemorrhage on T1-weighted images. Post-biopsy hemorrhage has low signal
intensity on T2-weighted images and can be indistinguishable from cancer.
On T1-weighted images, however, these foci present high signal intensity
and can thereby be differentiated from suspicious areas of low signal
intensity on T2-weighted images that represent cancer, therefore improving
the specificity of tumor nodule detection.
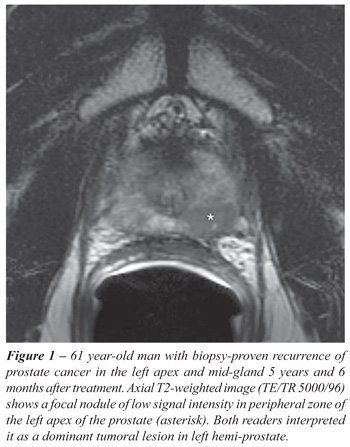
• Presence or absence of dominant
tumoral lesion on T2-weighted images. A study was considered positive
if a focal mass-like nodule or crescentic subcapsular focus of low T2
signal intensity was identified within the hemi-prostate (i.e., the left
or right side of the gland) (Figures-1 and 2). Because of the known limitations
of tumor localization and registration based on sextant biopsy results
(17,18), we localized tumor to the hemi-prostate. The limitation of the
prostatic sextant as a unit of analysis is illustrated in a prior study
of tumor localization with MR imaging and MR spectroscopic imaging, in
which the accuracy of imaging for sextant localization was only 67% (157
of 234) to 74% (173 of 234), but that of imaging for tumor lateralization
was 75% (80 of 106) to 88% (93 of 106) (19). The difference was, presumably,
at least partially due to errors in registration between imaged sections
and biopsy specimens. Such errors are likely to be magnified in the irradiated
gland because of radiation-induced shrinkage and distortion of tissue.

We opted to describe only the dominant lesion
in each patient based on the results of a study by Pucar et al. that demonstrated
that clinically significant local recurrence following radiation therapy
presents as a single focus at the site of primary tumor (20).
Standard
of Reference
Transrectal ultrasound-guided biopsy was
the standard of reference in this study. All but two biopsies were performed
at our institution using prostatic nerve blockade. The usual number of
specimens that were obtained is 16, using a systematic approach that targeted
the right and left sides of the gland at different levels, as well as
suspicious areas seen on ultrasound. We retrospectively reviewed the histopathological
reports of all procedures. A report was issued by one of the attending
pathologists in our institution for all cases, including the two performed
at an outside institution. Samples processed at our institution were fixed
in formalin immediately after biopsy and subsequently placed in a block
of paraffin wax. Microtome sections were then mounted on a glass slide
and stained with hematoxylin and eosin. High molecular weight keratin
immunoperoxidase staining was also performed on areas suspicious for adenocarcinoma.
Histopathological evidence of post-treatment effect only was considered
a negative result (21).
The presence of cancer on histopathology
reports was recorded on a per-sextant basis; however, for the reasons
stated above, we determined recurrent cancer to be absent or present in
the hemi-prostate.
Statistical
Analysis
When reading T2-weighted images, our study
design called for each reader to only identify the dominant side of a
lesion whenever it was bilateral (as explained previously within “Imaging
Interpretation”). Therefore, there was an inherent a priori constraint
to the data format that cancer could not be identified bilaterally. When
analyzing whether readers correctly diagnosed cancer, the definition of
the dominant side was taken into account according to the design given
in Table-1. That is, a positive diagnosis was considered correct if the
reader a) correctly diagnosed the patient as having cancer and if so;
b) correctly determined the side of the prostate gland containing cancer
- if the cancer was bilateral then the reader was considered correct regardless
of which side was named dominant. This allowed us to employ simple and
robust non-parametric statistical methods while also taking into account
whether the correct side of the prostate was diagnosed as containing cancer.

Kappa statistics were used to determine
the level of interobserver agreement.
For the purpose of statistical analysis,
the patients in this study were divided in two groups, “early”
and “late”. Patients who had imaging performed within the
first 3 years after external beam radiation therapy formed the group called
“early”. Conversely, the group named “late” included
all patients who were imaged three or more years after treatment. This
division was based on the results of the studies by Pollack and Vance
(4,5), which suggest that identification of cancer in the first two or
three years after treatment negatively impacts long-term outcome. Twenty-five
patients were imaged within 3 years of treatment and 34 more in the 3
years after therapy.
Because other factors may have inflenced
the accuracy of MR imaging, we assessed the similarity in distribution
of several variables between these two groups. The Wilcoxon signed rank
test was used to assess their distribution with respect to the continuous
variables of pre-treatment PSA level, Gleason score, and radiation dose.
Gleason score was treated as a continuous variable because of the large
number of possible categories and its ordinal quality. Fisher’s
exact test was used to assess the distribution of patients within the
two groups according to the discrete variables D’Amico risk stratification
(15), TNM stage, presence or absence of biochemical failure, and the use
of neoadjuvant or adjuvant hormonal therapy. The Freeman-Halton extension
of Fisher’s exact test was used for contingency tables larger than
2x2.
Logistic regression was used to test for
a difference in the accuracy of T2-weighted MR imaging for the detection
of cancer in these two groups. The logistic regression model included
group (“early” or “late”) and diagnosis (presence
or absence of cancer on biopsy). The primary test was used for an interaction
between group and diagnosis. A significant interaction would indicate
a difference in predictive accuracy depending on whether patients were
imaged early or late. The model was applied separately to each reader’s
data.
Statistical calculations were performed
using SAS/STAT? software v9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Histopathological
Findings
Forty-one hemi prostates (41/118, 34.7%)
in thirty-four patients (34/59, 57.6%) had evidence of cancer on histopathological
analysis of transrectal ultrasound-guided biopsy samples. Nineteen patients
had recurrence in the right side of the prostate, 8 in the left, and 7
bilaterally. Nine of these patients were part of early post-treatment
group (9/25, 36%) and 25 were part of the late post-treatment group (25/34,
73.5%). All seven patients with tumor detected on both sides of the prostate
were part of the latter group.
Patient
Characteristics
There were no statistically significant
differences in the balance of patients within groups “early”
and “late” according to pre-treatment PSA, clinical stage,
Gleason score, D’Amico’s risk stratification, radiation dose,
neoadjuvant and/or adjuvant hormonal therapy, and evidence of biochemical
failure at the time of imaging (Table-2).

MR
Imaging Results
None of the readers detected intra-prostatic
hemorrhage on T1-weighted MR images of 13 patients (13/59, 22.0%) who
underwent biopsy prior to imaging.
Overall, the diagnostic accuracy of T2-weighted
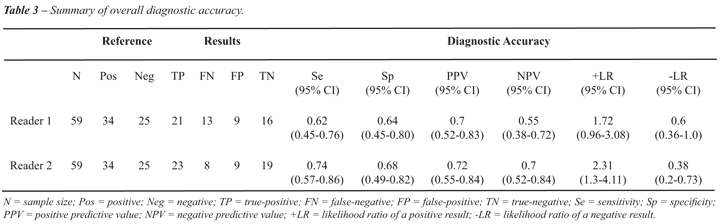
MR imaging after external beam radiation therapy was 63% (37/59), for
reader 1, and 71% (42/59), for reader 2. The sensitivity and specificity
of the method was 62% (21/34) and 64% (16/25), for reader 1, and 74% (23/31)
and 68% (19/28), for reader 2, respectively. These results, along with
the predictive values and likelihood ratios, are detailed in Table-3.
The interobserver agreement was considered
good on a per-patient and per-hemi-prostate basis (Kappa coefficient value
= 0.59 and 0.69, respectively).
The results of the diagnostic accuracy of
MR imaging per group, i.e. “early” and “late”,
are summarized in Table-4. For both readers, logistic regression failed
to demonstrate a statistically significant difference in the ability of
T2-weighted MR imaging to detect cancer based on whether patients were
imaged before or after 3 years (reader 1, p = 0.86; reader 2, p = 0.44).

DISCUSSION
Despite
our rather liberal criteria for a true positive outcome - identifying
tumor within a hemi-prostate, even if tumor was bilateral - the results
of our study suggest that T2-weighted endorectal MR imaging has low accuracy
for the detection of recurrent disease in patients who have undergone
definitive treatment with external beam radiation for prostate cancer.
The few published studies on MR imaging after EBRT have suggested T2-weighted
MR imaging has low to moderate accuracy for the detection of tumor after
radiation treatment (9-13). The variability in the numbers reported by
the different authors is mostly dependent on three factors: prevalence
of disease in the sample, sample size, and statistical analysis methodology.
Pucar et al. enrolled only nine patients,
all of which had known recurrence following radiation therapy. Using a
sextant approach, they found that MR imaging had a sensitivity of 68%
and specificity of 96%; however, they did not adjust for clustering effects
(10). Sala et al. reported areas under the receiver-operating curve (AU-ROC)
of 75% and 61%. They also reported the sensitivity and specificity of
MR imaging based on the dichotomization of results measured using a five-point
scoring system. These results were very similar to ours (sensitivity =
55-76%, specificity = 65-73%) (12). In a study that enrolled 22 patients,
Rouviere et al. reported a sensitivity ranging from 68% to 78% (11). Unfortunately,
all but three patients had recurrence, decreasing the significance of
the calculation of specificity. Coakley et al. included 21 patients in
their study and used the hemi-prostate as unit of analysis. Accounting
for clustering effects, they found an AU-ROC of 49% and 51% for MR imaging
(9). The study by Haider et al. also had a sample size (n = 49) and results
that were similar to ours, considering the 95% confidence intervals. According
to their study, MR imaging had a sensitivity and specificity of 58% and
52%, respectively (13).
The results of all above-mentioned studies,
including ours, suggest that MR imaging alone is insufficient for the
evaluation of such populations of patients and raises the question of
whether other imaging modalities should be used, separately or in conjunction
with T2-weighted MR imaging. Among the options available, multiparametric
endorectal MR imaging - an approach that incorporates other MR techniques,
such as MR spectroscopic imaging, dynamic enhanced MR imaging, and diffusion-weighted
MR imaging - is promising. Coakley et al. found that a combined approach
using MR imaging and MR spectroscopic imaging improved detection of tumor
(9). Both Haider and Rouviere reached similar conclusions when they investigated
the incremental value of dynamic enhanced MR imaging (11,13). Although
these studies support the use of multiparametric MR imaging in patients
treated with external beam radiation therapy, the results are preliminary
and further investigation with a larger, prospective trial is ultimately
required.
As a secondary analysis, we investigated
if the accuracy of the MR imaging was influenced by the time interval
between radiation treatment and MR imaging. This assumption was based
on observation of recovery of the usual zonal anatomy after radiation
and/or hormonal therapy and on the results of a study by Pickett et al.
(14) that showed recovery of normal metabolism at MR spectroscopic imaging
after treatment. We dichotomized the subjects in two groups, those whose
MR images were acquired within 3 years after treatment and those whose
imaging was performed after 3 years. This decision was supported by the
results presented by Pollack and Vance (4,5), which suggest that identification
of cancer in the first two or three years after treatment negatively impacts
long-term outcome. Our results did not demonstrate an influence of time
since treatment on accuracy of MR imaging on a logistic regression model.
It is unknown if this in fact represents an accurate picture of the situation
or just the result of insufficient power due to a small sample size.
It has been previously demonstrated that
hormonal deprivation therapy can significantly reduce tumor volume and
decrease peripheral zone signal on T2-weighted images (22), hence having
an additional influence in tumor detection on MR imaging. Although it
would be interesting to stratify patients in two groups (with and without
androgen deprivation therapy) to determine how this would affect our results,
it would not possible to obtain any meaningful results of accuracy due
to the small number of subjects in each subgroup. This is an issue that
must be addressed in future studies.
Our study has limitations. First, it was
a retrospective, single institution study. Our results probably are not
widely generalizable, as the expertise in MR imaging acquisition and interpretation
varies among institutions. Because of our retrospective research design,
we probably incurred a sample selection bias, as we included only patients
who had a transrectal ultrasound-guided biopsy. It may be expected that
the prevalence of recurrent cancer in our population is higher than in
the general population of patients treated for prostate cancer with external
beam radiation therapy. This could influence our results, as both positive
predictive value and negative predictive value are directly related to
the prevalence of disease. Although sensitivity and specificity would
not be affected. On the other hand, the indications of MR imaging after
radiation therapy have not yet been established and more likely the modality
will be added to the armamentarium used to investigate patients with suspected
local recurrence on the basis of clinical examination or PSA measurements.
In fact, our population is representative of this cohort and therefore
our results are useful for future standard procedure. Second, our sample
size is not large. This has two major effects on our results; it produces
a wide 95% confidence interval for diagnostic accuracy estimations and
does not provide us sufficient power to reject the null hypothesis - i.e.,
the interval of time between treatment and MR imaging does not affect
the detection of cancer with T2-weighted MR imaging - if this is fact
false (type II error). The wide confidence intervals explain the apparent
difference of accuracy between the two readers - not statistically significant
- despite relatively good interobserver agreement. Third, transrectal
ultrasound-guided biopsy is an imperfect standard of reference. The use
of an imperfect standard of reference results in bias of the estimated
error rates of MR imaging and the direction of this bias is usually downward
(23). In our study, which has a relatively large number of patients with
disease, i.e. positive biopsy, this bias is probably less significant
for the estimation of sensitivity than specificity. It is important, though,
to make clear that our results may overestimate the true accuracy of the
modality. In this setting, however, overestimation would in fact provide
further support to our conclusion: T2-weighted MR imaging appears to have
low accuracy for detection of recurrent cancer in patients who underwent
external beam radiation therapy. Although whole-mount histopathologic
analysis of salvage prostatectomy specimens may be considered a preferable
standard of reference, such surgery is infrequently performed in the population
we investigated. In addition, this approach also has limitations. In a
retrospective study, for instance, it may result in verification bias,
as the decision to proceed to surgery is likely influenced by positive
results of MR imaging. Our use of the hemi-prostate rather than the prostate
sextant as the unit of analysis might also be criticized, although as
noted above sextant localization is inaccurate when biopsy is compared
to radical prostatectomy specimens, likely due to errors in sextant localization
of ultrasound-guided biopsy needles. Such errors are likely to be even
greater in the shrunken post-radiation gland. Lateralization should be
less subject to such registration problems.
Lastly, the option to consider the reader
correct regardless of which side was named dominant in bilateral tumors
can also lead to incorrect higher accuracies of the imaging method. We
opted for this approach for two reasons: 1) this allowed us to employ
simple and robust non-parametric statistical methods while also taking
into account whether the correct side of the prostate was diagnosed as
containing cancer; and 2) detection of local recurrence in one side, even
if disease is bilateral, provides sufficient information for determining
management of these patients, as the current standard is to treat them
with salvage brachytherapy or salvage prostatectomy (+/- systemic therapy),
techniques that treat the entire gland. Irrespective, overestimation of
our results supports our conclusion.
In conclusion, T2-weighted MR imaging appears
to have low accuracy for detection of recurrent cancer in patients who
underwent external beam radiation therapy. Further and larger studies
are necessary to confirm these results and to determine if the interval
of time between treatment and MR imaging truly has no effect on the accuracy
of the method.
ACKNOWLEDGEMENT
Dr. Antonio C. Westphalen is supported by NIBIB T32 Training Grant 1 T32 EB001631, and RSNA Research & Education Foundation 2006-07 Research Fellow Grant #FEL0602 and 2007-2009 Research Scholar Grant #RSCH0709
CONFLICT OF INTEREST
None declared.
REFERENCES
- Stephenson RA, Stanford JL: Population-based prostate cancer trends in the United States: patterns of change in the era of prostate-specific antigen. World J Urol. 1997; 15: 331-5.
- Kestin LL, Vicini FA, Ziaja EL, Stromberg JS, Frazier RC, Martinez AA: Defining biochemical cure for prostate carcinoma patients treated with external beam radiation therapy. Cancer. 1999; 86: 1557-66.
- Moul JW: Prostate specific antigen only progression of prostate cancer. J Urol. 2000; 163: 1632-42.
- Pollack A, Zagars GK, Antolak JA, Kuban DA, Rosen II: Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002; 54: 677-85.
- Vance W, Tucker SL, de Crevoisier R, Kuban DA, Cheung MR: The predictive value of 2-year posttreatment biopsy after prostate cancer radiotherapy for eventual biochemical outcome. Int J Radiat Oncol Biol Phys. 2007; 67: 828-33.
- Rouvière O: MR assessment of recurrent prostate cancer after radiation therapy. Radiology. 2007; 242: 635-6; author reply 636-7.
- Chan TW, Kressel HY: Prostate and seminal vesicles after irradiation: MR appearance. J Magn Reson Imaging. 1991; 1: 503-11.
- Coakley FV, Hricak H, Wefer AE, Speight JL, Kurhanewicz J, Roach M: Brachytherapy for prostate cancer: endorectal MR imaging of local treatment-related changes. Radiology. 2001; 219: 817-21.
- Coakley FV, Teh HS, Qayyum A, Swanson MG, Lu Y, Roach M 3rd, et al.: Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology. 2004; 233: 441-8.
- Pucar D, Shukla-Dave A, Hricak H, Moskowitz CS, Kuroiwa K, Olgac S, et al.: Prostate cancer: correlation of MR imaging and MR spectroscopy with pathologic findings after radiation therapy-initial experience. Radiology. 2005; 236: 545-53.
- Rouvière O, Valette O, Grivolat S, Colin-Pangaud C, Bouvier R, Chapelon JY, et al.: Recurrent prostate cancer after external beam radiotherapy: value of contrast-enhanced dynamic MRI in localizing intraprostatic tumor--correlation with biopsy findings. Urology. 2004; 63: 922-7.
- Sala E, Eberhardt SC, Akin O, Moskowitz CS, Onyebuchi CN, Kuroiwa K, et al.: Endorectal MR imaging before salvage prostatectomy: tumor localization and staging. Radiology. 2006; 238: 176-83.
- Haider MA, Chung P, Sweet J, Toi A, Jhaveri K, Ménard C, et al.: Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 70: 425-30.
- Pickett B, Kurhanewicz J, Coakley F, Shinohara K, Fein B, Roach M 3rd: Use of MRI and spectroscopy in evaluation of external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004; 60: 1047-55.
- D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al.: Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280: 969-74.
- Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al.: Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006; 65: 965-74.
- Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J, et al.: Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000; 164: 400-4.
- Obek C, Louis P, Civantos F, Soloway MS: Comparison of digital rectal examination and biopsy results with the radical prostatectomy specimen. J Urol. 1999; 161: 494-8; discussion 498-9.
- Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, et al.: Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging--clinicopathologic study. Radiology. 1999; 213: 473-80.
- Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al.: Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007; 69: 62-9.
- Kestin LL, Goldstein NS, Vicini FA, Mitchell C, Gustafson GS, Stromberg JS, et al.: Pathologic evidence of dose-response and dose-volume relationships for prostate cancer treated with combined external beam radiotherapy and high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002; 54: 107-18.
- Padhani AR, MacVicar AD, Gapinski CJ, Dearnaley DP, Parker GJ, Suckling J, et al.: Effects of androgen deprivation on prostatic morphology and vascular permeability evaluated with mr imaging. Radiology. 2001; 218: 365-74.
- Hawkins DM, Garrett JA, Stephenson B: Some issues in resolution of diagnostic tests using an imperfect gold standard. Stat Med. 2001; 20: 1987-2001.
____________________
Accepted after revision:
December 12, 2008
_______________________
Correspondence address:
Dr. Antonio Carlos Westphalen
Abdominal Imaging
University of California San Francisco
505 Parnassus Avenue, Box 0628, M-372
San Francisco, California, 94143-0628, USA
Fax: + 1 415 476-0616
E-mail: antonio.westphalen@radiology.ucsf.edu
EDITORIAL COMMENT
The
detection of locally recurrent prostate cancer, after external radiation
therapy (EBRT), is essential since further treatment options are variable.
This includes additional irradiation of the prostate, hormonal therapy,
salvage prostatectomy and other new treatment options such as cryosurgery
and transrectal high-intensity focused ultrasound. Although several treatment
options are available, the management of recurrent prostate cancer after
EBRT is a difficult task since all these modalities are associated with
high risks of complication (1). For these reasons, precise detection of
local recurrence of the tumor is of utmost importance for the management
of these patients. The authors performed a retrospective study in order
to determine the accuracy of T2-weighted endorectal MR imaging in the
detection of prostate cancer after EBRT, and also to investigate the relationship
between imaging accuracy and time since therapy. They concluded that “T2-weighted
endorectal MR imaging has low accuracy in the detection of prostate cancer
after external beam radiation therapy, irrespective of the time since
therapy”.
As pointed out by the authors in the introduction
of their manuscript, tumor depiction with conventional endorectal magnetic
resonance imaging in the irradiated gland is of limited value due to treatment-related
changes that include prostatic shrinkage, diffuse low T2 signal intensity
in the gland, and indistinctness of the normal zonal anatomy (2,3). Since
irradiated prostate gland usually appears small and diffusely hypointense
on T2-weighted images, magnetic resonance spectroscopic imaging (MRSI),
which depicts abnormal metabolism rather than abnormal anatomy, has been
shown to be much better technique for the detection of local tumor recurrence
and for the demonstration of complete metabolic atrophy (4-6). At our
institution, in the last 5 years, we have been using a comprehensive protocol
for the detection of recurrent disease in patients treated with EBRT.
This protocol consists of a combination of conventional endorectal T2-weighted
image with multiparametric functional MRI studies (MRSI, dynamic contrast
enhanced and diffusion-weighted images). Using the transrectal guided
biopsy as reference, similarly to the authors, we have so far found greater
accuracy when using this protocol as compared with conventional T2-weighted
images (7).
Regarding the influence of time after EBRT,
we found that serial MR spectroscopic imaging is also superior to convention
endorectal MRI to demonstrate areas of normal or abnormal metabolism,
which can be observed several months after the end of EBRT. Further studies,
however, are warranted to confirm this hypothesis.
REFERENCES
- Rouvière O: MR assessment of recurrent prostate cancer after radiation therapy. Radiology. 2007; 242: 635-6; author reply 636-7.
- Coakley FV, Hricak H, Wefer AE, Speight JL, Kurhanewicz J, Roach M: Brachytherapy for prostate cancer: endorectal MR imaging of local treatment-related changes. Radiology. 2001; 219: 817-21.
- Chan TW, Kressel HY: Prostate and seminal vesicles after irradiation: MR appearance. J Magn Reson Imaging. 1991; 1: 503-11.
- Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, et al.: Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging--clinicopathologic study. Radiology. 1999; 213: 473-80.
- Yu KK, Scheidler J, Hricak H, Vigneron DB, Zaloudek CJ, Males RG, et al.: Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999; 213: 481-8.
- Coakley FV, Teh HS, Qayyum A, Swanson MG, Lu Y, Roach M 3rd, et al.: Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology. 2004; 233: 441-8.
- van Dorsten FA, van der Graaf M, Engelbrecht MR, van Leenders GJ, Verhofstad A, Rijpkema M, et al.: Combined quantitative dynamic contrast-enhanced MR imaging and (1)H MR spectroscopic imaging of human prostate cancer. J Magn Reson Imaging. 2004; 20: 279-87.
Dr.
Adilson Prando
Chief, Department of Radiology
Vera Cruz Hospital
Campinas, São Paulo, Brazil
E-mail: adilson.prando@gmail.com
EDITORIAL COMMENT
Detection
of post-treatment recurrence of prostate cancer is a challenging situation,
both after radical prostatectomy and radiation therapy, since PSA alone
may not differentiate between biochemical, local and/or systemic recurrence.
Endorectal MRI (E-MRI), given its intrinsic
high contrast resolution, would be the ideal imaging exam for non-invasive
detection of local recurrence. However, T2-weighted images of the prostate
(the standard imaging technique for prostate MRI) may not suffice for
the detection of recurrence, especially after radiation therapy.
The article from Dr. Westphalen et al. reemphasizes
the limitations of T2-weighted MRI for the detection of local recurrence
after radiation therapy, regardless of the time interval between the procedure
and the imaging study.
It should be kept in mind, however, that
these results certainly do not diminish the value of E-MRI for the purpose
of local recurrence detection. Recent studies have shown that the use
of complimentary MRI techniques (namely, spectroscopy and contrast-enhanced
dynamic MRI) significantly increases accuracy of the method for the detection
of local recurrence, both after radical prostatectomy and after radiation
therapy (1,2). Moreover, a recent article correlating MRI and step-section
pathology demonstrated that clinically significant local recurrence after
radiation therapy occurs at the same site of the primary tumor, so the
use of E-MRI before and after treatment could lead to early detection
of local recurrence suitable to salvage therapy (3).
Therefore, we can conclude that E-MRI, when
used appropriately with the correct dedicated techniques, should be considered
in the diagnostic workflow of patients with suspected local recurrence
after prostate cancer treatment.
REFERENCES
- Pucar D, Sella T, Schöder H: The role of imaging in the detection of prostate cancer local recurrence after radiation therapy and surgery. Curr Opin Urol. 2008; 18: 87-97.
- Haider MA, Chung P, Sweet J, Toi A, Jhaveri K, Ménard C, et al.: Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 70: 425-30.
- Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al.: Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007; 69: 62-9.
Dr.
Ronaldo Hueb Baroni
Institute of Radiology
University of São Paulo, USP
São Paulo, SP, Brazil
E-mail: rbaroni@einstein.br