THE
ROLE OF NEPHRECTOMY OF THE ATROPHIC KIDNEY IN BEARERS OF RENOVASCULAR
HYPERTENSION
(
Download
pdf )
doi: 10.1590/S1677-55382010000200005
MYRIAN J. THOMAZ, ANTONIO M. LUCON, JOSE N. PRAXEDES, LUIS A. BORTOLOTTO, MIGUEL SROUGI
Department of Urology, Faculty of Medicine, University of Sao Paulo, Sao Paulo, Brazil
ABSTRACT
Purpose:
Evaluation of the beneficial effect of nephrectomy of the atrophic kidney
on blood pressure (BP) and renal function.
Materials and Methods: A retrospective study of 51 patients with renovascular
hypertension (RVH), bearers of atrophic kidney due to severe stenosis
or occlusion of the renal artery. Average age was 47.1 ± 15 years,
the median creatinine clearance was 54 mL/min, average systolic BP (SBP)
149.6 ± 22.5 mm Hg, average diastolic BP (DBP) 90.8 ± 17
mm Hg and the median number of hypotensors 3 (1 to 5) per patient per
day. Blood pressure and serum creatinine were analyzed from 12 to 60 months
after the nephrectomy.
Results: There was a significant improvement in the average SBP in the
periods from 12 to 36 months (p = 0.028) and for the average DBP from
12 to 48 months after the nephrectomy (p = 0.045), accompanied by a significant
reduction in the use of hypotensors from 12 to 48 months (p < 0.05).
One year after the nephrectomy, there was a 69% improvement in blood pressure
and 63.8% improvement in renal function of patients.
Conclusion: The removal of atrophic kidney in patients with RVH is a safe
procedure which presents benefits for the control of arterial hypertension
and renal function in bearers of renovascular hypertension.
Key
words: nephrectomy; atrophic; kidney renovascular; hypertension
Int Braz J Urol. 2010; 36: 159-70
INTRODUCTION
Among the
causes of secondary hypertension, we emphasize renovascular hypertension
resulting from renal ischemia caused by the total or partial stenosis
of the renal artery, presenting an atherosclerotic etiology in 90% of
the cases and fibrodysplasia of the artery in 10% (1,2). It is the second
most common cause of secondary hypertension, only after parenchymal renal
diseases. It occurs in from 1 to 5% of the adult hypertensive population,
but increases in the elderly, diabetics and bearers of systemic atherosclerotic
lesions (1,3). It has growing participation as a cause of dialytic renal
insufficiency (4,5).
Ischemic nephropathy is caused by the chronic and hemodynamically significant
occlusion of the renal artery which results in a considerable reduction
in the blood flow, a diminution in the glomerular filtration rate and
leads to the progressive damage of the renal parenchyma (1,3,6). The renal
hypoflow results in the activation of the renin-angiotensin-aldosterone
(RAA) system, responsible in the final analysis for vascular alterations,
damage to the dependent endothelial vasodilation and an increase in oxidative
stress, mainly in the etiology of atherosclerotic patients (7).
Renovascular hypertension (RVH) can be cured in special cases. The revascularization
of the ischemic kidney, whether surgical or by percutaneous angioplasty
(PTA), is the best means of treatment and should be considered when pharmacological
treatment is insufficient for the adequate control of the blood pressure
or when inexplicable renal insufficiency appears. We analyzed the nephrectomy
of the atrophic kidney (less than 8 cm) in those patients with refractory
blood pressure or a deterioration of the renal function, when the revascularization
of the artery was no longer possible.
MATERIALS AND METHODS
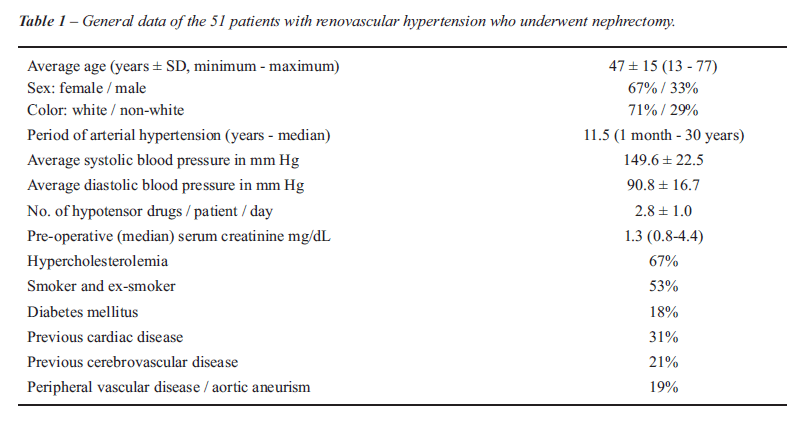
This was a retrospective observational study of 61 patients with a diagnosis of RVH who underwent nephrectomy during the period from January 1989 to January 2007. Ten patients were lost to follow-up. The general data of the patients is found in Table-1. The average age of the patients was 47.1 ± 15 years with a predominance of the female sex and white. The average pre-operative SBP was 149.6 ± 22 mm Hg and the average pre-operative diastolic blood pressure (DBP) was 90.8 ± 16.7 mm Hg with 2.8 ± 1 hypotensors per patient per day. The median pre-operative serum creatinine was 1.3 mg /dL (0.8 to 4.5 mg/dL) analyzed by the colorimetric method (8). Hypercholesterolemia and smoking were the most frequent risk factors.
The average renal size of the atrophic kidney
was 7.1 ± 1.1 cm (10 to 5.2 cm) and the contralateral kidney was
10.9 ± 0.9 cm (9.3 to 13.5 cm), measured by ultrasonography. On
echo-Doppler examination of the renal arteries the stenosis which made
up = 70% of the diameter of the blood vessel were considered as hemodynamically
significant lesions. The function of the atrophic kidney measured by renal
scintigraphy with the radioisotope DTPA and captopril was 8% (0-49%).
The measurement of the plasma rennin activity
(PRA) was performed by radioimmunoassay Gamma Coat PRA I125 Ria Kit, by
the collection of blood by selective catheterization of the renal veins
and cava inferior.
Of the 35% (18/51) of patients who were
bearers of significant bilateral lesion, 17.6% (9/51) required revascularization
by percutaneous transluminal angioplasty (PTA) of the contralateral artery.
The previous surgical revascularization by means of an autotransplant
of the contralateral kidney was carried-out in 9.8% (5/51) of the patients,
4% (2/51) of whom no longer required dialysis. Eight percent (4/51) did
not undergo any procedure.
The arteriography, performed by means of
selective catheterization of the renal arteries, was performed in 74%
(38/51) of the cases. The magnetic resonance angiography of the renal
arteries was performed in 16% (8/51) of the patients and its principal
indicator was the presence of creatinine equal to or greater than 2 mg/dL.
The recommendation for the nephrectomy of
the atrophic kidney was made only after total or significant occlusion
of the renal artery, associated with the absence of renal function, had
been demonstrated. In 70.5% of the patients nephrectomies by video laparoscopy
were performed and in the remaining 29.5% nephrectomies by lumbotomy.
The postoperative measurements of blood
pressure were made in accordance with the recommendations of the WHO.
and were completed each 12 months for five years, with the same period
for the collection of serum creatinine. Patients were regarded as cured
who had DBP < 90 mm Hg and systolic (SBP) < 140 mm Hg with no hypotensor
medication and with an improvement in blood pressure with DBP < 90
mm Hg and /or SBP < 140 mm Hg with the same or a reduction in hypotensor
medication or with a reduction in DBP of at least 15 mm Hg with the same
number or a reduction in hypotensor medication (9). We considered therapeutic
failures those cases in which there was a worsening of blood pressure
or an increase in the number of hypotensors for the same pressure levels;
all the other cases benefited from the treatment, in accordance with the
criteria cited above (9).
In the assessment of renal function, we
used the estimated creatinine clearance, obtained on the basis of the
MDRD equation (modification of diet in renal disease study prediction
equation) (9,10). The numbers obtained were transferred to the creatinine
clearance graph where each line represents a patient throughout the period
of observation, the horizontal and ascending lines indicating an improving
and the descending ones, a worsening renal function.
Statistical Analysis
The data
have been summarized as numbers and percentages for the qualitative variables
and as averages ± standard deviations where the supposition of
normality of the data has been satisfied and in those cases for which
this supposition could not be made, the median was used (minimum - maximum).
The assessment of the pressure levels over the study period was performed
by means of a variance analysis model (ANOVA) and the measurements repeated,
the multiple comparisons were submitted to the construction of contrasts.
The comparison between the groups ( R x NR) (Responders x Non-Responders)
or Improvement x Worsening) was made by means of the Chi-squared test
when the variable was qualitative and in those cases in which the value
expected was less than five, the Fisher’s Exact Test was used. For
the quantitative variables Student’s-t-test or Mann’s test
was used for those cases in which the supposition of normality could not
be made.
A significance level of 5% was considered significant. SAS (Statistical
Analysis System V. 9.0) software was used for the analysis of the data.
RESULTS
There was statistically significant variation both for the SBP (p < 0.001) and for the DBP (p = 0.005) over time (Figure-1). The decrease observed at 12 months post nephrectomy was 14.5 ± 2.8 mm Hg (p < 0.001) for the SBP and 9.7 ± 2.4 mm Hg (p < 0.001) for DBP. From 12 months to 60 months postoperatively there was no statistically significant variation whether in SBP (p > 0.05) or DBP (p > 0.05), shown in the respective Tables-2 and 3. It was observed that the average decrease in SBP from the pre-nephrectomy period to each of the periods assessed was significant until 36 months post-nephrectomy, while the average decrease in the DBP presented statistical significance until 48 months as compared with that of the pre-nephritic period (Table-4).
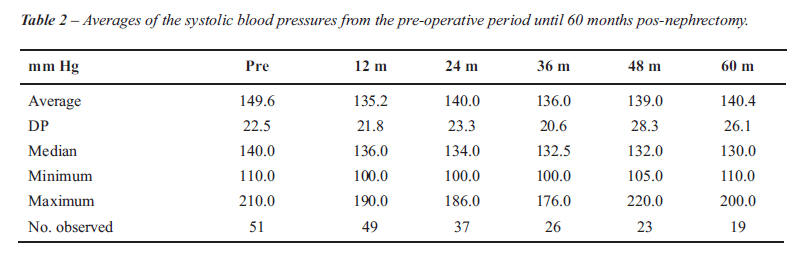
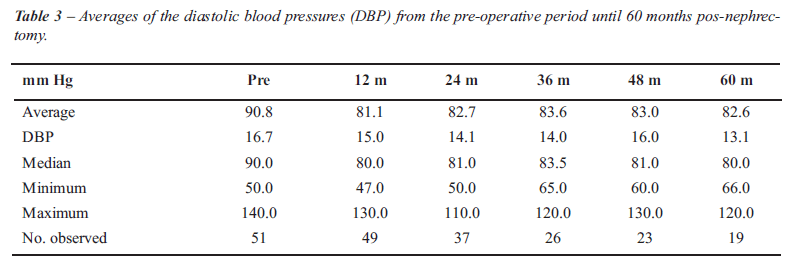
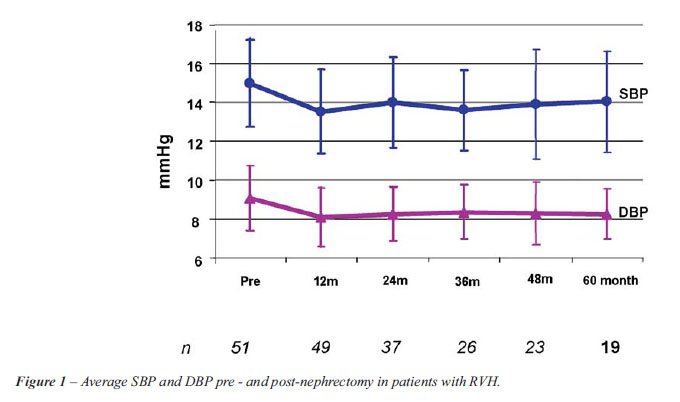
The average number of hypotensors used in
the pre-operative period of 2.8 hypotensors/day diminished significantly
(p < 0.05) for the period of 12 months and remained constant until
48m (2.0 hypotensors/day) after the nephrectomy.
One year after the nephrectomy 49 patients
were still being followed-up and the BP of 69% of them had improved, in
accordance with the criteria previously mentioned (9). There was one death
and one dialytic renal insufficiency (2/51), which were excluded from
the sample. The value of the variations of the DBP and SBP at 12 months
for each patient are shown in Figures-2 and 3 respectively, where in the
lower part of each figure are shown the patients whose pressure levels
improved. There was an 8% (4/51) cure rate of RVH, 3 of the patients had
fibromuscular dysplasia of the renal artery.

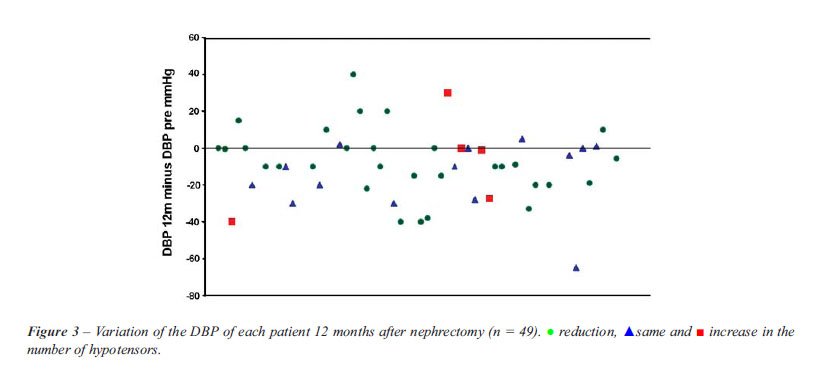
Sixty-nine percent (34/49) of the patients
were considered responders as they had shown an improvement in blood pressure
and 30.6% (15/49) of the patients as non-responders as their blood pressure
had worsened. There was no significant difference (p > 0.05) between
the groups for any of the factors analyzed: age, sex, color, initial blood
pressure, initial creatinine, bilateral disease, risk factors and co-morbidities
(Table-5). The renal function of the patients was checked by the measurement
of serum creatinine taken every 12 months up to 60 months post-nephrectomy
and the estimated creatinine clearance (MDRD) was calculated. There was
a follow-up loss of 7.8% (4/51) of the patients.
Figure-4 shows the progress in renal function
over the 60 months of observation. Twelve months post-nephrectomy 64%
(30/47) of the patients presented an improvement in creatinine clearance
(MDRD), represented by the ascending curves.
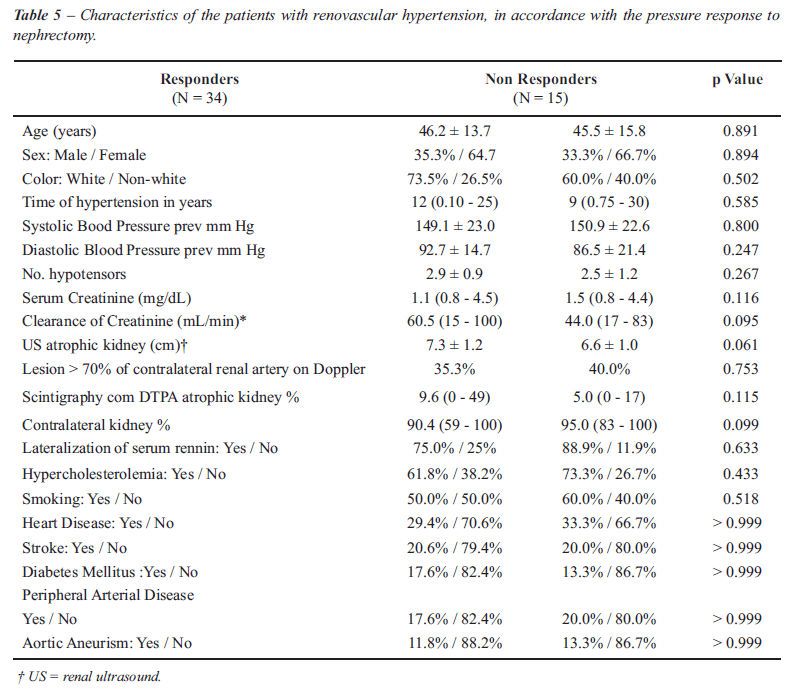
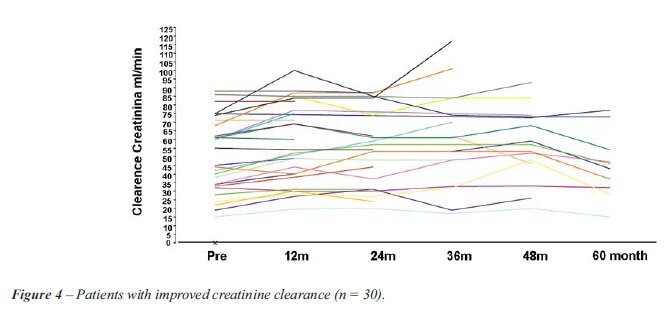
Figure-5 shows the progress in renal function
over 60 months. One can observe the 36% (17/47) of patients whose renal
function worsened (falling curves). Worthy of note among this group are
the 23% of patients who initially lost renal function, but who demonstrated
recovery of this function during the follow-up period. Dialysis was necessary
in 8% (4/51) of the patients over the 60-month period.
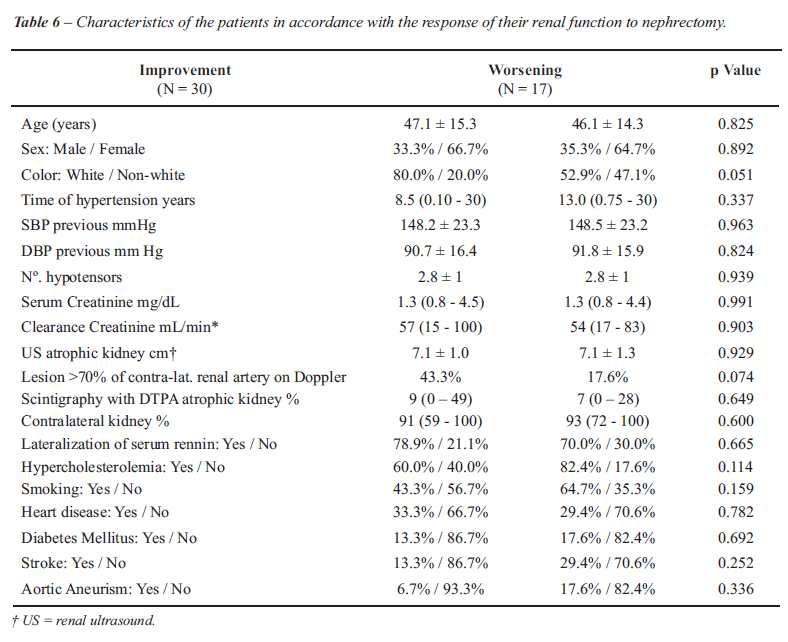
When the characteristics of the patients
divided into groups according to the improvement or worsening of their
renal function were studied, they were found to be homogeneous, that is
to say, without any statistically significant difference among the variables
studied (p > 0.05), i.e., these same factors as were analyzed for the
blood pressure (Table-6).
Sixty percent of the cases (31/49) of this
study had levels of serum rennin activity. Lateralization of the rennin
was found in 85% of the patients divided among the different groups, with
no statistical significance.
In eleven patients (21.5%) there was no
segmental stenosis of the vessel. In the histopathological analysis of
the renal artery of the remaining patients we found atherosclerosis of
the renal artery in 63% of the cases, fibromuscular dysplasia in 26% and
Takayasu arteritis in 6%. The histological data obtained from the renal
parenchyma are: ischemic atrophy of the renal parenchyma in 72% of the
cases, benign nephrosclerosis in 38%; malignant nephrosclerosis in 10%,
ischemic infarction of the renal parenchyma in 4% of the patients, interstitial
nephritis in 6%, chronic pyelonephritis in 12 % and malignant renal neoplasms
(of less than 1 cm) in 4% of the cases studied.
In the group with improvement of the renal
function, 54.2% of the patients had a diagnosis of fibromuscular dysplasia
and 29.2% of the cases had atherosclerosis and by means of the application
of the generalization of Fisher’s Exact Test, a statistically significant
association was observed (p = 0.003) between this histological type and
the improvement in renal function. No embolic complications were identified
in this series.
COMMENTS
RVH treatment
has changed over recent years. The use of nephrectomy has declined as
a result of the rise of new hypotensor drugs, developed on the basis of
the knowledge the aldosterone angiotensin rennin system and the development
of angioplasty techniques (PTA) with the implanting of “stents”
(3,11). More recently, the improved understanding of the mechanisms which
harm dependent endothelial dilation and oxidative stress, especially as
related to high levels of angiotensin II, particularly in the atherosclerotic
lesion, herald new forms of treatment for the future (12).
The removal of the atrophic kidney caused by the severe stenosis of the
renal artery was carried-out in patients with refractory arterial hypertension
with no possibility of revascularization.
Various studies have been reported in the literature comparing percutaneous
revascularization and conservative clinical treatment in the treatment
of RVH (13), but there are few series regarding the nephrectomy of the
atrophic kidney as an alternative form of treatment for the bearer of
renovascular hypertension with blood pressure of difficult control (14-16).
This study analyzed 51 nephrectomies of the atrophic kidney for complete
obstruction of the renal artery. There was a predominance of the video
laparoscopic technique, which was used in 70.5% of the cases. A year after
nephrectomy, SBP was 135 ± 21 mm Hg and DBP of 81 ± 15 mm
Hg, significantly lower than the initial levels, and they were thus maintained
for 36 months and 48 months, respectively, together with a significant
reduction in the number of hypotensors.
The division into groups on the basis of response in terms of blood pressure
and renal function one year after nephrectomy, was performed to identify
those characteristics which determined the response. Sixty-eight percent
of the cases which presented an improvement in arterial hypertension were
included in the group (R), 8% of them being cured after one year, 75%
(3/4) of them being patients with RHV due to fibromuscular dysplasia of
the renal artery and 32% of the remaining cases belonging to the NR group
(Table-5). The creatinine clearance (MDRD) curve (9,10,13) was used to
enable us to classify the patients by group into those which presented
either improvement or worsening of the renal function at 12 months. There
was an improvement in creatinine clearance one year after nephrectomy
in 64% of the cases (Figure-4) and a worsening in 36% (Figure-5). Twelve
percent of the patients with creatinine clearance < 30 mL/min at surgery,
continued not to need dialysis during the period of 60 months analyzed
(Figure-4) and 8.5% of the patients whose renal function worsened at 12
months, showed recovery of the function over the 60 months (Figure-5).
There was no statistically significant lesion present (Table-6). Moderate
renal insufficiency, which might be an important factor leading to a final
need for dialysis, was not significant in this patient population, possibly
because of the small number of cases involved.
In the four groups, the characteristics studied, such as sex, color, age,
smoking, hypercholesterolemia and co-morbidities presented similar distribution
with no significant statistical difference (p > 0.095). No lateralization
of the rennin was found as a predicative factor of results, similar data
are described by Hasbak et al. (17). The factors which would explain the
absence of the lateralization of rennin are bilateral stenosis of the
renal artery, states of volemic depletion, chronic use of inhibitors of
the conversion enzyme of angiotensin I to II (IECA) and renal insufficiency.
Rossi et al. (18) found a correlation of the lateralization of serum rennin
activity only in bearers of RVH with unilateral atrophic kidney by complete
occlusion of the renal artery that underwent nephrectomy, but there was
no lateralization of the rennin plasma in patients treated with angioplasty.
The presence of significant lesion (> 70%) of the contra-lateral renal
artery made no statistically significant difference (p = 0.753) to the
results of this study. Bilateral lesion was treated by nephrectomy after
revascularization of the contra-lateral renal artery in 27% (14 patients).
Kane et al. (14) also found an improvement in blood pressure in patients
who had undergone nephrectomy of the atrophic kidney (59%) for the treatment
of RVH. In the comparison, in nephrectomy with the revascularization of
the ischemic kidney undertaken by Oskin et al. (13) to evaluate blood
pressure and renal function, nephrectomy was as effective as revascularization
for the control of arterial hypertension, but was inferior to revascularization
for the preservation of the renal function.
The average age of 47 years in this series is inferior to the 65 years
of other reported series (3,13,14). Of the 34 (67%) women in this study,
38% were of childbearing age, at risk of becoming pregnant. The prevalence
of renovascular hypertension during pregnancy is unknown, whereas the
significantly greater risk of pre-eclampsia in the woman with chronic
arterial hypertension is known. Nephrectomy of the atrophic kidney before
a new pregnancy after a failure, can improve the maternal and fetal prognosis
(19).
The relationship between stenosis of the renal artery and other systemic
lesions is well known (20-22). The significant prevalence of stenosis
of the renal artery (greater than 75%) in patients who died from stroke
was 10.4% of the cases, 4.6% of whom with complete occlusion of the vessel
(20). In the literature, patients with peripheral vascular disease associated
with stenosis of the atherosclerosclerotic renal artery suffered higher
mortality from cardiac causes during the two-year follow-up (21) and the
severity of the lesion of the renal artery was directly proportional to
the worst cardiac prognosis (22).
The exact relationship between the degree of stenosis and the loss of
mass is unknown. As the Doppler is not an invasive examination it has
been used for the follow-up of stenotic lesions. Significant stenosis
(> 60%) and severe arterial hypertension, progressed with 36% of renal
atrophy over two years, followed-up using Doppler examination(23).
Arteriography was the examination primarily used to determine the presence
of the obstruction and the viability of the atrophic kidney. Presently,
magnetic resonance angiography and angiotomography of the renal arteries
may be used as alternatives to arteriography with the advantage of avoiding
arterial catheterization (24,25). However, when there is a suspicion of
fibrodysplasia of the renal artery, where the lesions are more distal,
magnetic resonance angiography is not appropriate, as it has greater sensitivity
to the lesions of the artery located up to 3 cm from the ostium (24,25).
In this series, among the causes of the renal atrophy which led to nephrectomy,
we found atherosclerosis of the renal artery, fibromuscular dysplasia,
trauma of the renal artery, thrombosis of the renal artery in patients
submitted to renal autotransplant and failure of endovascular revascularization.
In another series, the nephrectomy of the atrophic kidney cured the RVH
caused by a fall which occurred when removing the traumatic lesion from
the renal artery (26). The high incidence of thrombosis is due to the
fact that the renal arteries in these cases are small, difficult to manipulate
and possess lesions aggravated by previous procedures (16).
The diagnosis of fibromuscular dysplasia of the renal artery was associated
with better results as regards both pressure and renal function, renal
dialytic insufficiency being a rare cause (27).
From the physiological point of view, the reduction in the levels of angiotensin
II after the nephrectomy of the atrophic kidney would cause lesser oxidative
stress and could lead to the restoration of the dependent endothelial
dilation, responsible in the final analysis for the vascular lesions (renal,
coronary and cerebral), mainly those of atherosclerotic etiology. It is
known that high levels of angiotensin II are related to an increase in
the pro-fibrotic factors, with the consequent replacement of the functioning
glomerulus by fibrosis, leading to a loss of renal mass and the advance
of renal insufficiency. Hypercholesterolemia, especially the increase
in the oxidized LDL, associated with stenosis of the renal artery, speeds
up the development of fibrosis in the ischemic kidney by the spread of
pro-fibrotic mechanisms (NF-ß, TGF-ß, oxidative stress among
others) and suppression of the remodeling of the tissues (28).
In the future, we should have more options for the treatment of arterial
hypertension and vascular obstruction. New therapies such as gene therapy
and cell therapy, which do not call for the use of drugs, are appearing.
The implantation of embryonic cells (stem cells) or of genes which codify
angiogenic factors may be used as alternative therapies for the inoperable
vascular obstructions. Gene therapy takes endothelial growth factors or
blocks the harmful genes involved in the pathogenesis of the disease.
The endothelial dysfunction caused by oxidative stress, an important mechanism
of the atherosclerotic vascular lesion, may be treated in the future either
by cell therapy or by gene therapy in association with pharmacological
therapy (29,30).
CONCLUSION
In patients with renovascular hypertension, nephrectomy of the atrophic kidney is a procedure which results in improvement of the arterial hypertension and of the renal function in two-thirds of patients.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Safian RD, Textor SC: Renal-artery stenosis. N Engl J Med. 2001; 344: 431-42.
- Slovut DP, Olin JW: Fibromuscular dysplasia. N Engl J Med. 2004; 350: 1862-71.
- Garovic VD, Textor SC: Renovascular hypertension and ischemic nephropathy. Circulation. 2005; 112: 1362-74.
- Rimmer JM, Gennari FJ: Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med. 1993; 118: 712-9.
- Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, et al.: Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002; 36: 443-51.
- García-Donaire JA, Alcázar JM: Ischemic nephropathy: detection and therapeutic intervention. Kidney Int Suppl. 2005; 68: S131-6.
- Loscalzo J: Nitric oxide and vascular disease. N Engl J Med. 1995; 333: 251-3.
- Seelig HP: The Jaffe reaction with creatinine. Z Klin Chem Klin Biochem. 1969;:18: 30-9.
- Rundback JH, Sacks D, Kent KC, Cooper C, Jones D, Murphy T, et al.: Guidelines for the reporting of renal artery revascularization in clinical trials. American Heart Association. Circulation. 2002; 106: 1572-85.
- Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, et al.: Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant. 2006; 6: 100-8.
- Gray BH: Intervention for renal artery stenosis: endovascular and surgical roles. J Hypertens Suppl. 2005; 23: S23-9.
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K: Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002; 346: 1954-62.
- Krumme B, Donauer J: Atherosclerotic renal artery stenosis and reconstruction. Kidney Int. 2006; 70: 1543-7.
- Oskin TC, Hansen KJ, Deitch JS, Craven TE, Dean RH: Chronic renal artery occlusion: nephrectomy versus revascularization. J Vasc Surg. 1999; 29: 140-9.
- Kane GC, Textor SC, Schirger A, Garovic VD: Revisiting the role of nephrectomy for advanced renovascular disease. Am J Med. 2003; 114: 729-35.
- Geyskes GG, Oei HY, Klinge J, Kooiker CJ, Puylaert CB, Dorhout Mees EJ: Renovascular hypertension: the small kidney updated. Q J Med. 1988; 66: 203-17.
- Hasbak P, Jensen LT, Ibsen H; East Danish Study Group on Renovascular Hypertension: Hypertension and renovascular disease: follow-up on 100 renal vein renin samplings. J Hum Hypertens. 2002; 16: 275-80.
- Rossi GP, Cesari M, Chiesura-Corona M, Miotto D, Semplicini A, Pessina AC: Renal vein rennin measurements accurately identify renovascular hypertension caused by total occlusion of renal artery. J Hypertension. 2002; 5: 975-84.
- Thorsteinsdottir B, Kane GC, Hogan MJ, Watson WJ, Grande JP, Garovic VD: Adverse outcomes of renovascular hypertension during pregnancy. Nat Clin Pract Nephrol. 2006; 2: 651-6.
- Kuroda S, Nishida N, Uzu T, Takeji M, Nishimura M, Fujii T, et al.: Prevalence of renal artery stenosis in autopsy patients with stroke. Stroke. 2000; 31: 61-5.
- Pillay WR, Kan YM, Crinnion JN, Wolfe JH; Joint Vascular Research Group, UK: Prospective multicentre study of the natural history of atherosclerotic renal artery stenosis in patients with peripheral vascular disease. Br J Surg. 2002; 89: 737-40.
- Conlon PJ, Little MA, Pieper K, Mark DB: Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001; 60: 1490-7.
- Caps MT, Zierler RE, Polissar NL, Bergelin RO, Beach KW, Cantwell-Gab K, et al.: Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int. 1998; 53: 735-42.
- Pedersen EB: New tools in diagnosing renal artery stenosis. Kidney Int. 2000; 57: 2657-77.
- Lee V: The 89 th. Annual Scientific Assembly and Annual Meeting of Radiological Society of North America. Renal MRI: Novel techniques and contrast agents.2006 [Access in 2006 Jan 06]. Available at: www.medscape.com
- Negoro H, Iwamura H, Oka H, Kawakita M, Ariyoshi K, Koda Y, et al.: Traumatic renal artery thrombosis with renovascular hypertension. Int J Urol. 2004; 11: 903-5.
- Mounier-Vehier C, Lions C, Jaboureck O, Devos P, Haulon S, Wibaux M, et al.: Parenchymal consequences of fibromuscular dysplasia renal artery stenosis. Am J Kidney Dis. 2002; 40: 1138-45.
- Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, et al.: Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003; 23: 1295-301.
- Higashi Y, Nishioka K, Umemura T, Chayama K, Yoshizumi M: Oxidative stress, endothelial function and angiogenesis induced by cell therapy and gene therapy. Curr Pharm Biotechnol. 2006; 7: 109-16.
- Melo LG, Gnecchi M, Pachori AS, Kong D, Wang K, Liu X, Pratt RE, Dzau VJ: Endothelium-targeted gene and cell-based therapies for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004; 24: 1761-74.
____________________
Accepted after revision:
October 18, 2009
_______________________
Correspondence address:
Dr. Myrian J. Thomaz
Department of Urology
Faculty of Medicine, University of Sao Paulo
Sao Paulo, Brazil
E-mail: m.j.thomaz@uol.com.br