PROPHYLAXIS
AND THERAPEUTIC EFFECTS OF RASPBERRY (RUBUS IDAEUS) ON RENAL STONE FORMATION
IN BALB/C MICE
(
Download pdf )
IBRAHIM F. GHALAYINI, MOHAMMED A. AL-GHAZO, MOHAMMAD N. A. HARFEIL
Urology Division, King Abdullah University Hospital, Jordan University of Science and Technology - Irbid, Jordan
Basic and Translational Urology
Vol. 37
(2): 259-267, March - April, 2011
doi: 10.1590/S1677-55382011000200013
ABSTRACT
Purpose:
To evaluate the prophylactic potential of herbal decoction from Rubus
idaeus, a medicinal plant widely used in the Middle East to treat kidney
stones, by assessing the effect of administration in experimentally induced
calcium oxalate (CaOx) nephrolithiasis in mice.
Materials and Methods: This study was based
on administration of glyoxylate and/or herbal treatments simultaneously
for 12 days, followed by histological and biochemical tests. Group I was
used as a negative control. Group II was only given daily intra-abdominal
injection of glyoxylate (80 mg/Kg). Group III and IV were given 100 mg/kg/day
and 200 mg/kg/day of aqueous extract of R. idaeus by gavage, respectively
in addition to glyoxylate injection. To examine the effect of anti-oxidants
on hyperoxaluria-induced changes in kidney, the enzymatic and non-enzymatic
anti-oxidant levels were assessed.
Results: Significant reductions were obtained
in the urinary oxalate, calcium and phosphorus values in the herbal-treated
groups relative to untreated animals while creatinine excretion increased.
Serum oxalate, calcium and creatinine were significantly reduced, while
phosphorus was not significantly changed. Kidney content of calcium was
higher in the untreated group. Mice in treated groups at 12 days had significantly
more superoxide dismutase, catalase, glutathione reductase (GSH) and G6PD
activities than the untreated group. Hyperoxaluria-induced generation
of malondialdehyde (MDA) and protein carbonyls was significantly prevented
in the treated groups. R. idaeus had a significantly high content of vitamin
E in the herbal treated groups. The histology showed more CaOx deposition
in the kidneys of untreated animals.
Conclusion: Rubus idaeus has an impressive
prophylactic effect on CaOx stones in nephrolithic mice. There is a possible
role of lipid peroxidation in CaOx stone formation which may has a relationship
with the major risk factors in urine including oxalate, calcium, phosphorus
and MDA. Further experimental studies are required to elucidate the chemical
constituents of the active ingredients of this interesting plant.
Key
words: kidney stones; Rubus idaeus; glyoxylates; calcium oxalate
Int Braz J Urol. 2011; 37: 259-67
INTRODUCTION
The
incidence of kidney stones has increased in the last five decades, in
association with economic development. Most calculi in the urinary system
arise from a common component of urine, e.g. calcium oxalate (CaOx), representing
up to 80% of analyzed stones (1,2). Kidney stone formation consists of
several stages including supersaturation, nucleation, growth, aggregation,
and retention within renal tubules (3). The recurrence of urolithiasis
represents a serious problem, as patients who have formed a stone are
more likely to form another, and thus stone prevention is highly recommended.
The introduction of new techniques for removing stones including extracorporeal
shockwave lithotripsy (ESWL) has improved the management of urolithiasis,
but recent studies show that, apart from the high cost that ESWL entails,
exposure to shock waves, even in therapeutic doses, is associated with
several adverse effects, including renal injury, decrease in renal function,
and more importantly an increase in stone recurrence (4). Thus, more efforts
are needed to better assess medical therapy and to develop new agents
that can be used either alone or combined to prevent stone formation more
efficiently with fewer side-effects. Our attention is particularly on
phytotherapy, which is common in traditional medicine as an alternative
to primary healthcare in many countries. Some herbs show efficient cure
of urinary stones like Phyllanthus niruri and Hernaria hirsuta (5-7).
Others support the use of traditional Chinese medicine Kampo herbal and
Acupuncture in stone disease management in which antilithic beneficial
effects include increased urinary volume, inhibitory activity of Ca oxalate
aggregation and inhibition of Ca oxalate nucleation (8).
Raspberry (Rubus idaeus), that belongs to
Rosaceae family, is a commercial fruit crop widely grown in all template
regions of the world. R. idaeus is very vigorous and can be invasive.
They propagate using basal shoots, extended underground shoots that develop
roots and individual plants. It is widely distributed in the Mediterranean
countries and used in folk medicine in Jordan, Syria and Palestine to
treat renal stones. In the present study, the capability of R. idaeus
young roots was investigated as a therapeutic agent for preventing kidney
stone formation in a mouse model of hyperoxaluria.
MATERIAL AND METHODS
Preparation of Extracts
Rubus idaeus young roots were collected from Na’or city which is near the capital Amman during the month of May 2007. It was identified and stored by Professor Dawoud Asawi, the plant Taxonomist in the Herbarium division of the Department of Biology at Jordan University. Here, 200 g of young roots were extracted in a Soxhlet extraction apparatus (ACMS technocracy, India) using distilled water and concentrated on a rota evaporator. The resultant filtrate was lyophilized and the lyophilizate was stored at -20°C in desiccants until used. The average (w/w) yield was 11.5% (mother extract).
Mouse Model for Stone
Formation
Study of all animals followed the recommendations of the NIH Guide for the Care and Use of Laboratory Animals. To induce CaOx kidney stones in mouse, glyoxylate which is oxalate precursor, was introduced using the previously reported method in rat experimental nephrolithiasis models (9). Intra-abdominal injection was performed according to the weight of each mouse. Forty-eight C57BL/6 male mice (8 weeks old), weighing 25-30 g were divided equally into 4 groups of 12 mice each. All except the control group were administered 80 mg/kg glyoxylate by daily intra-abdominal injection. The adopted administration method used in this study was optimized by preliminary experiments according to Okada et al. (10). All animals had free access to drinking water (ad libitum) and regular chow every day and were kept under a controlled 12 hours light/dark cycle at 22 ± 2°C. Water and food intake were measured for all groups.
Herbal Treatment
The animals were divided into 4 groups. Group I was used as a negative control (not supplemented with glyoxylate or herbal treatment). Group II was only given daily intra-abdominal injection of glyoxylate (80 mg/Kg) as mentioned before. Group III and IV were given 100 mg/kg/day and 200 mg/kg/day of aqueous extract of R. idaeus young roots by gavage in addition to glyoxylate injection, respectively. All the mice were fed on a standard laboratory diet and weighed daily. The experiment was conducted for the following 12 days. Then, the mice underwent the following tests: serum tests, urine tests, calcium determination of kidneys, and renal histology. Serum and urine tests were repeated three times at the time of sacrifice using different samples.
Serum Tests
At the end of the experiment, each mouse was anesthetized by an intraperitoneal injection of urethane (2 g/kg body weight). Blood was recovered from all animals for analysis of serum calcium, oxalate, phosphorus and creatinine determined with an automatic analyzer after centrifugation. The % of reduction was calculated for the different parameters using the formula: (mean values of untreated animals - mean values of treated animals) × 100 / mean values of treated animals.
Detection of Kidney
Stone Formation
The animals’ right kidneys were removed and cut longitudinally. Renal specimens were fixed in 4% paraformaldehyde, and embedded in paraffin. Four micrometer-thick cross-sections were stained with the previously described Pizzolato staining method to detect oxalate-containing crystals (11). Briefly, paraffin sections were dewaxed and rinsed in distilled water. Hydrogen peroxide (30%) and silver nitrate (5%) were mixed equally, 1 mL each, and poured onto the slides with tissue sections (pH of this mixture is 6.0). Each slide was exposed to light from a 60-W incandescent lamp at a distance of 15 cm (6 in.) for 15-30 min. The slides were washed thoroughly with distilled water and stained with safranin and then dehydrated in the usual manner. Thin sections were prepared for tissue histology, including the renal papilla and the existence and the frequency of crystal deposition in the renal tissue was observed in each group by light microscopy.
Calcium Determination of Kidneys
The left kidneys were removed from the mice for calcium determination. The kidneys were dried at 100°C for 24 hours and weighed. They were minced in a beaker to which 7 mL of 0.5 N nitric acid was added. The beaker was then heated until the liquid became transparent. After calibration using the standard calcium solution, the calcium content was determined by atomic absorption spectroscopy. The calcium content of the kidney was expressed as mg/g wet tissue of the kidney (12).
Oxidative Stress
Markers
of oxidative stress were malondialdehyde content (MDA), representing lipid
peroxidation (LPO) determined by the thiobarbituric acid reactive method
(13). Protein carbonyls were measured according to the method of Levine
et al. (14). Antioxidants composed of vitamin E, determined by the method
of Arnuad et al., using High performance liquid chromatography (15). Superoxide
dismutase (SOD) was measured as described by Misra and Fridovich (16),
and catalase using the method of Sinha (17). Glutathione content analyzed
by the method of Tietze (18), and glucose-6-phosphate dehydrogenase (G6PD)
activity was determined according to the method of Deutsch (19).
The results from all groups were statistically compared using Student’s-t-test,
with P < 0.05 considered to indicate significant differences. Data
were presented as mean ± standard deviation.
RESULTS
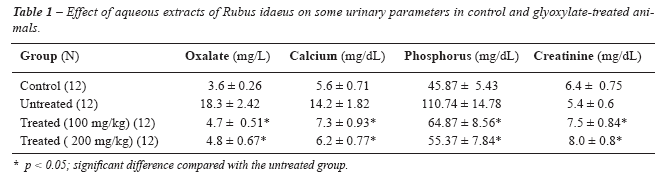
Table-1
shows that statistically significant reductions were obtained in the urinary
oxalate, calcium and phosphorus values in the herbal-treated groups relative
to untreated animals (P < 0.05). The reductions were 289.4%, 94.5%
and 70.7%, in oxalate, calcium, and phosphorus, respectively. In contrast,
creatinine excretion increased in the treated groups. Excretion of all
tested parameters was approximately similar in both herbal-treated groups.
There was no significant increase in the volume of water intake or food
ingestion between all groups (p < 0.05).
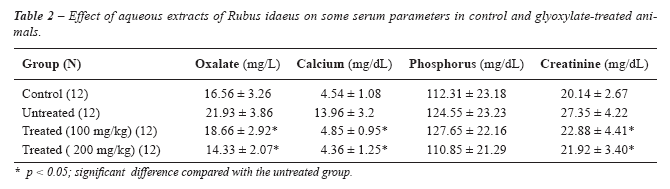
Table-2 shows that serum oxalate, calcium and creatinine were significantly
reduced (17.5%, 187.8% and 19.5%, respectively) (P < 0.05), while phosphorus
was not significantly changed. No significant difference was obtained
among the two herbal-treated groups.
The weight of each kidney of untreated animals
was significantly higher (0.27 ± 0.04 g) than that of treated animals
(0.19 ± 0.03 g and 0.18 ± 0.03 g, for the 100 g/Kg and 200
g/Kg R. idaeus, respectively) (p < 0.05).
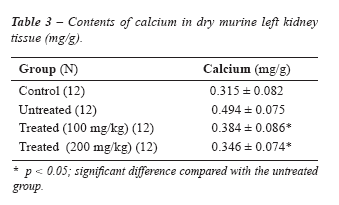
Kidney content of calcium is shown in Table-3
for the different mice groups. It is significantly higher in the untreated
group than the others (P < 0.05).
To examine the effect of anti-oxidants on
hyperoxaluria-induced changes in kidney, the enzymatic and non-enzymatic
anti-oxidant levels were assessed in the blood of all animals (Table-4).
Mice in group III and IV at 12 days had significantly more SOD, catalase,
glutathione reductase (GSH) and G6PD activities than in the herbal-untreated
group (group II) (p < 0.05).
Kidney tissue peroxidation was estimated
as MDA level and protein carbonyls were assessed as an indicator of protein
peroxidation products (Table-4). Mice in the treated groups III and IV
had significantly less MDA levels and protein carbonyls than in group
II (p < 0.05). Hyperoxaluria-induced generation of MDA and protein
carbonyls was significantly prevented in group III and IV (P < 0.05).
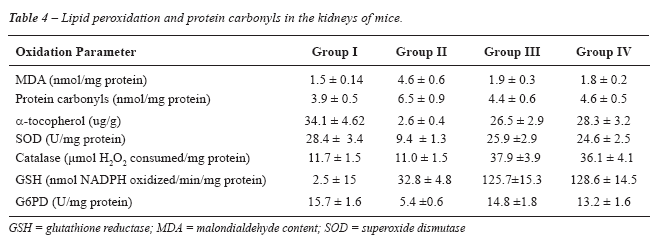
To find out whether vitamin E might play
a protective role against hyperoxaluria-induced renal peroxidative damage,
the kidney tissue alpha-tocopherol levels were also measured. As shown
in Table-4, R. idaeus had a significantly higher content of vitamin E
in the herbal treated groups III and IV in contrast to group II.
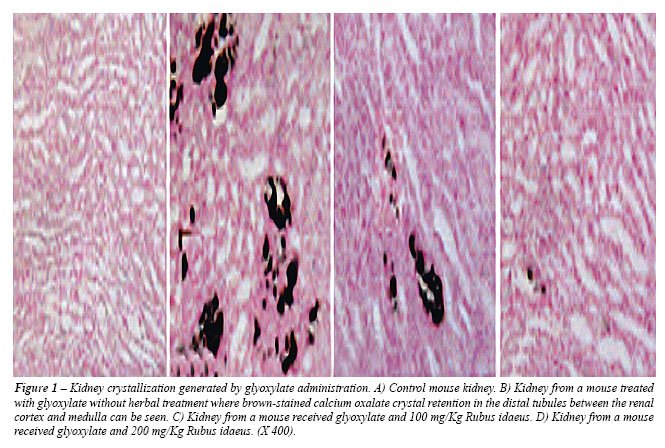
The histology also showed more CaOx deposition in all parts of the kidney
of untreated mice (Figure-1B) but almost no deposition in the negative
control and treated mice (Figure-1A, C and D). However, deposition was
less in higher dose treated mice (Figure-1D) than in the lower dose treated
mice (Figure-1C).
COMMENTS
Phytotherapy
is common in folk medicine as an alternative to primary healthcare in
many countries. R. idaeus is a plant belonging to Rosaceae family with
a worldwide distribution. The leaves have been used for centuries as a
folk medicine to treat canker sores, cold sores, and gingivitis in persons
of all ages as well as to treat anemia, leg cramps, diarrhea, and morning
sickness in pregnant women, and as a uterine relaxant.
From the testimony of herbalists and patients
with lithiasis, the plant is widely known for its ability to aid in expelling
stones from the urinary tract after a few days of treatment. To our knowledge,
the efficacy of R. idaeus in treating urolithiasis has not been evaluated
previously. Accordingly, we undertook the present study to assess the
effectiveness of R. idaeus as a prophylactic agent for CaOx stones in
experimentally induced nephrolithiasis in mice.
In this study, males were chosen because earlier studies have shown that
the amount of stone deposition in male mice was significantly more common
(20). In response to 12 day period of glyoxylate administration, young
mice formed renal calculi composed mainly of CaOx (9,10).
The administration of a small volume of
aqueous R. idaeus extract induced a significant reduction in calculus
growth and in some animals even the CaOx seed almost not found, suggesting
that these animals eliminated the CaOx matrix in the absence of any modification
in diuresis rate.
The administration of R. idaeus decreased
the urinary excretion of elements that act as calculus promoters, including
calcium and oxalate, although the serum levels of these elements were
decreased, suggesting that R. idaeus interferes with the tubular transport
of these substances with association of an unknown route that decrease
serum levels. Thus the inhibition of calculus growth was independent of
alterations in the urinary and serum concentrations of these lithogenic
elements. It is important to measure 24 hours urine output to clarify
this point. Further research is required to detect the mechanism of action
of R. idaeus and the route of excretion of these lithogenic elements.
Therefore, the plant extract may contain substances that inhibit crystal
growth, leading to the production of small particles, as the animals continued
to receive glyoxylate. At death, all the kidneys from the untreated mice
showed gross hypertrophy with large crystalline deposits in all parts,
but those from treated mice were apparently of normal size, with few and
smaller particle deposits, and in most cases none. Thus the extract of
R. idaeus could have substances that eliminate pre-existing stones, although
an increase in urinary oxalate would then be expected in treated mice,
which was not apparent. The fate of the excess of oxalate is uncertain;
we suggest, like others, that changes in the inhibitors of stone formation,
including citrate, magnesium (Mg) and glycosaminoglycans (GAGs), may bind
oxalate in the gut, reducing its intestinal absorption, although a role
for substances in the plant extract cannot be excluded (7,21).
Freitas et al. investigated the effect of
an aqueous extract of Phyllanthus niruri (Pn), a plant used in folk medicine
to treat lithiasis, on the urinary excretion of endogenous inhibitors
of lithogenesis, citrate, Mg and GAGs (7). Their results showed that Pn
has an inhibitory effect on crystal growth, which was independent of changes
in the urinary excretion of citrate and Mg, but might be related to the
higher incorporation of GAGs into the calculi.
Atmani and Khan, investigated the effectiveness
of an extract obtained from Herniaria hirsuta on CaOx crystallization
in vitro (22). The nucleation and aggregation of CaOx crystals were measured
separately using spectrophotometric methods. Results showed that there
were more crystals with increasing concentration of extract but that they
were proportionally smaller. They concluded that extract of H. hirsuta
promoted the nucleation of CaOx crystals, increasing their number but
decreasing their size.
In another study, Atmani et al. suggested that H. hirsute may even contain
substances that dissolve pre-existing particles (6), which we are investigating
with the R. idaeus extract in a study which is under process. All trials
for the achievement of relatively large stones in mice and rats models
failed, and therefore, we could not claim that R. idaeus extract dissolves
or disaggregates the CaOx crystals. Further study of the effect of R.
idaeus extract on the interaction of CaOx crystals with renal epithelial
cells in culture is needed to evaluate the mechanism by which crystal
deposits were eliminated. Also, experimental studies are required to elucidate
the chemical constituents of the active ingredients of this interesting
plant.
Similar to other authors, we found that
tubular enzyme (GSH), urinary oxalate and Calcium levels seemed to have
positive and significant correlations with lipid peroxides (MDA) in animals
with CaOx crystals deposition (23). Huang et al. evaluated the possible
role of lipid LPO in CaOx stone formers, and determined the relationship
of LPO with the major risk factors in urine, including oxalate, citric
acid, calcium, phosphorus, Mg, osteopontin (OPN) and MDA (24). They concluded
that LPO correlated with hyperoxaluria and renal tubular damage, indicating
that hyperoxaluria can induce tubular cell injury. Oxalate-induced membrane
injury was mediated by LPO reaction through the generation of oxygen free
radicals (24). In urolithic animal kidney or oxalate exposed cultured
cells; superoxide anion is generated in excess, causing cellular injury
(23). The LPO products were excessively released in tissues of urolithic
animals. The accumulation of these products was concomitant with the decrease
in SOD, catalase, and G6PD as well as vitamin E, and reduced glutathione
(GSH). All the above parameters were decreased in urolithic condition
in our study, which was similarly, mentioned by others, irrespective of
the agents used for the induction of urolithiasis (23). LPO positively
correlated with calcium level and negatively correlated with GSH and vitamin
E. Antioxidant therapy to urolithic animals with vitamin E, glutathione
monoester, or fish oil may normalize the cellular antioxidant system,
enzymes and scavengers, and interrupt membrane lipid and protein peroxidation
reaction and its associated calcium accumulation. R. idaeus had a significant
high content of vitamin E in the herbal treated groups in this study.
Some authors proved that antioxidant therapy can prevent CaOx precipitation
in the rat kidney and reduced oxalate excretion in stone patients (23-26).
Similarly, CaOx crystal deposition in vitro to urothelium was prevented
by free radical scavengers such as phytic acid and mannitol by protecting
the membrane from free radical-mediated damage. Thamilselvan and Menon
demonstrated in-vivo evidence that hyperoxaluria-induced peroxidative
injury induces individual CaOx crystal attachment in the renal tubules
(26,27). They concluded that excess vitamin E completely prevented CaOx
deposition, by preventing peroxidative injury and restoring renal tissue
antioxidants and glutathione redox balance.
In conclusion, R. idaeus has a potent prophylactic
effect on CaOx stone formation, confirming the folklore about its anti-lithiasis
activity. There is a possible role of lipid peroxidation in CaOx stone
formation which may has a relationship with the major risk factors in
urine, including oxalate, calcium, phosphorus and MDA. It seems that antioxidant
therapy can prevent CaOx precipitation in the kidney and reduced oxalate
excretion in stone individuals. Therefore, vitamin E content might provide
protection against the deposition of CaOx stones in the kidney of humans.
Further experimental studies are required to elucidate the chemical constituents
of the active ingredients of this interesting plant.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Finlayson B: Symposium on renal lithiasis. Renal lithiasis in review. Urol Clin North Am. 1974; 1: 181-212.
- Khan SR: Structure and development of calcific urinary stones. In Bonucci E, (ed.), Calcification in Biological Systems. Boca Raton, CRC Press. 1992; pp.345-63.
- Khan SR: Interactions between stone-forming calcific crystals and macromolecules. Urol Int. 1997; 59: 59-71.
- Bellakhdar J, Claisse R, Fleurentin J, Younos C: Repertory of standard herbal drugs in the Moroccan pharmacopoea. J Ethnopharmacol. 1991; 35: 123-43.
- Hennequin C, Lalanne V, Daudon M, Lacour B, Drueke T: A new approach to studying inhibitors of calcium oxalate crystal growth. Urol Res. 1993; 21: 101-8.
- Atmani F, Slimani Y, Mimouni M, Hacht B: Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003; 92: 137-40.
- Freitas AM, Schor N, Boim MA: The effect of Phyllanthus niruri on urinary inhibitors of calcium oxalate crystallization and other factors associated with renal stone formation. BJU Int. 2002; 89: 829-34.
- Miyaoka R, Monga M: Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol. 2009; 35: 396-405.
- Khan SR: Experimental calcium oxalate nephrolithiasis and the formation of human urinary stones. Scanning Microsc. 1995; 9: 89-100; discussion 100-1.
- Okada A, Nomura S, Higashibata Y, Hirose M, Gao B, Yoshimura M, et al.: Successful formation of calcium oxalate crystal deposition in mouse kidney by intraabdominal glyoxylate injection. Urol Res. 2007; 35: 89-99.
- Pizzolato P: Histochemical recognition of calcium oxalate. J Histochem Cytochem. 1964; 12:333-6.
- Economou C. Thomus J, tombelem G, Arvis G: Predominance gauche de la lithiase renale. Sem Hop Paris. 1987; 63: 277-80.
- Buege JA, Aust SD: Microsomal lipid peroxidation. Methods Enzymol. 1978; 52: 302-10.
- Levine RL, Williams JA, Stadtman ER, Shacter E: Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994; 233: 346-57.
- Arnaud J, Fortis I, Blachier S, Kia D, Favier A: Simultaneous determination of retinol, alpha-tocopherol and beta-carotene in serum by isocratic high-performance liquid chromatography. J Chromatogr. 1991; 572: 103-16.
- Misra HP, Fridovich I: The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 247: 3170-5.
- Sinha AK: Colorimetric assay of catalase. Anal Biochem. 1972; 47: 389-94.
- Tietze F: Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969; 27: 502-22.
- Deutsch J: Glucose-6-phosphate dehydrogenase. In Bergmeyer HV (ed.), Methods in Enzymatic Analysis. 3rd edn. Volume 3. New York, Academic Press. 1983; pp. 190-7.
- Prasad KV, Bharathi K, Srinivasan KK: Evaluation of Musa (Paradisiaca Linn. cultivar)--”Puttubale” stem juice for antilithiatic activity in albino rats. Indian J Physiol Pharmacol. 1993; 37: 337-41.
- Osswald H, Weinheimer G, Schutt D: Effective prevention of calcium oxalate crystal formation in vitro and in vivo by pentosan polysulfate. In Walker VR, Sutton RAL, Cameron ECB, Pak CYC, Robertson WG. (ed.), Urolithiasis. New York, Plenum Press. 1989: pp. 141-4.
- Atmani F, Khan SR: Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in vitro. BJU Int. 2000; 85: 621-5.
- Selvam R: Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res. 2002; 30: 35-47.
- Huang HS, Ma MC, Chen CF, Chen J: Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology. 2003; 62: 1123-8.
- Huang HS, Ma MC, Chen J: Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am J Physiol Renal Physiol. 2009; 296: F34-45.
- Thamilselvan S, Menon M: Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 2005; 96: 117-26.
- Huang
HS, Chen J, Chen CF, Ma MC: Vitamin E attenuates crystal formation in
rat kidneys: roles of renal tubular cell death and crystallization inhibitors.
Kidney Int. 2006; 70: 699-710. Erratum in: Kidney Int. 2007; 71: 712.
____________________
Accepted
after revision:
July 30, 2010
_______________________
Correspondence
address:
Dr. Ibrahim Fathi Ghalayini
Professor of Urology
P.O. Box 940165
Amman, 11194, Jordan
Fax: +00 962 6568-7422
E-mail: ibrahimg@just.edu.jo
EDITORIAL COMMENT
Herbal
medicine has been used as an alternative to treat kidney stones in different
regions of the world for centuries. As we dove into the era of evidence
based medicine it is of utmost importance that technical and objective
beneficial effects can be demonstrated for this line of therapy facilitating
its acceptance and practical use (1).
Rubus ideaus in this elegant work from Ghalayini et al. was shown to be
able to not only reduce the amount of lithogenic constituents in mice
urine (notably oxalate), but also to eliminate the pre existent calculi
matrix even when animals were kept on continuous glyoxylate injection.
Treating hyperoxaluria is not always effective as patients struggle with
diet restrictions and fail to adhere to adequate medical therapy such
as pyridoxine administration (2).
Rubus idaeus may prove to be a suitable
alternative as it can be ubiquitously found and especially if decoction
could be achieved in a homemade fashion. Clinical trials may provide this
answer along with the determination of the ideal dose for humans, its
impact on 24-hour urine analysis and presence of any side effects.
REFERENCES
- Miyaoka R, Monga M: Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol. 2009; 35: 396-405.
- Ortiz-Alvarado O, Miyaoka R, Kriedberg C.: Pyridoxine and dietary counseling for the management of idiopathic hyperoxaluria in stone formers. Urology. 2011 [Epub ahead of print]
Dr. Ricardo
Miyaoka
Department of Urologic Surgery
University of Minnesota
1420 Delaware St. SE, (MMC 394)
Minneapolis MN 55455, USA
E-mail: miyao002@umn.edu