EFFICACY AND SAFETY
OF SILDENAFIL CITRATE FOR THE
TREATMENT OF ERECTILE DYSFUNCTION IN LATIN AMERICA
S. GLINA, E. BERTERO, J. CLARO, R. DAMIÃO, G. FARIA, A. FREGONESI, J. JASPERSEN, A. MENDOZA, D. MATTOS JR., L.C. ROCHA, M. SOTOMAYOR, C. TELÕKEN, S. URETA, E. ZONANA, F. UGARTE
Albert Einstein Hospital; HSPE; EPM; São Paulo, Brazil; UERJ, Rio de Janeiro, Brazil; Clinica Rio Claro, SP, Brazil; Unicamp, Campinas, SP, Brazil; Hospital General de México, Hospital Médica Sur, México, HC, Curitiba, PR, Brazil, Instituto Nacional de Nutrición Salvador Zubirán, México, Santa Casa de Porto Alegre, Porto Alegre, RS, Brazil, Hospital Español de México, Hospital Central Militar, México, Hospital Angeles del Pedregal, México
ABSTRACT
In
this 12-week, double blind, randomized, placebo-controlled, multicenter,
parallel group, flexible-dose study of oral sildenafil, 245 patients were
treated in 15 centers in Latin America (9 in Brazil and 6 in Mexico).
Patients began treatment with a 50-mg dose of sildenafil or matching placebo
that could be adjusted to 100 mg or 25 mg based on efficacy and tolerability.
One hundred twenty-four patients were randomized
to receive sildenafil and 121 to receive placebo. The most common concomitant
medical condition was unspecified essential hypertension in 24% and 29%
of the patients in the placebo and sildenafil groups, respectively. Scores
for the 2 primary efficacy variables, frequency of penetration and frequency
of maintained erection (International Index of Erectile Function Question
3 and Question 4), were both significantly greater in the sildenafil group
(p < 0.001) compared with the placebo group. The number of patients
who felt the study drug had improved their erections (36% for placebo
vs. 81% for sildenafil) and the mean proportion of successful attempts
at sexual intercourse during the last 4 weeks (32% for placebo vs. 71%
for sildenafil) was also significantly higher for patients receiving sildenafil
(p < 0.001).
Sildenafil was well tolerated with only
1 discontinuation due to a treatment-related adverse event (headache).
Mild headache and flushing were the most frequently reported adverse events.
None of the 6 serious adverse events (5 in the placebo group vs. 1 in
the sildenafil group) was considered to be related to treatment. In conclusion,
sildenafil is a well tolerated and effective treatment for erectile dysfunction
of psychogenic, organic, or mixed etiology in Latin American men.
Key words:
erectile dysfunction; penile erection; impotence; treatment
Braz J Urol, 27: 148-154, 2001
INTRODUCTION
Viagra®
(sildenafil citrate) was the first oral drug approved for the treatment
of erectile dysfunction (ED) (1). It was approved in Brazil and Mexico
on February 5, 1998, and April 29, 1998, respectively. At the present
time, it is approved in more than 100 countries worldwide and available
in more than 80 countries.
The safety and efficacy of sildenafil has
been shown in clinical trials that were conducted primarily in the United
States and Europe (1,2). Since the initial clinical trials, additional
studies have been conducted in different countries ranging from Africa
to Asia, but final results have not yet been published.
The prevalence of ED in South America is
expected to increase 150% from the year 1995 to 2025 (3). This will result
in a projected increase in the number of men with ED from 10.5 million
to 26.1 million. Thus, a need exists for an effective and acceptable treatment
for ED in Latin America. The objective of the present clinical trial was
to evaluate the safety and efficacy of oral sildenafil administered over
a 12-week period to Latin American men with ED of organic, mixed, and
psychogenic etiology.
METHODS
Study
Design
This was a double-blind, randomized, placebo-controlled,
multicenter, parallel-group, flexible-dose study of oral sildenafil taken
approximately 1 hour before sexual intercourse. The study was conducted
at 15 clinics (6 in Mexico and 9 in Brazil). The total duration of the
study was 16 weeks, with a 4-week run-in period and 12 weeks of drug treatment.
Patients visited the clinic at screening (week - 4), at the start of the
study (week 0), and at weeks 2, 4, 8, and 12. All patients began treatment
with sildenafil 50 mg, which could be increased to 100 mg for lack of
efficacy or decreased to 25 mg if adverse events occurred.
Patients
Approximately 250 patients were enrolled
who met the following requirements: 1)- aged 18 years or more, 2)- documented
clinical diagnosis of ED of more than 6 months’ duration, 3)- in
a stable relationship with a female partner for at least 6 months, and
4)- gave written informed consent. Patients were excluded if they had
1)- genital anatomic deformities; 2)- hormonal abnormalities (high prolactin
or low testosterone levels); 3)- a major psychiatric disorder that was
not well controlled by treatment; 4)- known history of alcoholism or substance
abuse; 5)- history of major hematologic, renal, or hepatic abnormalities;
6)- ED following a spinal cord injury; 7)- diabetes with either poor glycemic
control or untreated proliferative diabetic retinopathy; 8)- history of
stroke, myocardial infarction, or significant cardiovascular disease within
the previous 6 months; 9)- hypotension (blood pressure < 90/50 mm Hg)
or hypertension (blood pressure > 170/100 mm Hg); 10)- concurrent use
of nitrates or nitric oxide donors; and 11)- known history of retinitis
pigmentosa.
The intent-to-treat population consisted
of all the patients who were randomized, took at least 1 dose of study
medication, and had at least 1 post randomization evaluation of the efficacy
variable in question. The efficacy evaluated population consisted of all
the patients who were randomized, completed the study, and answered the
primary efficacy questions.
Efficacy
Assessments
The efficacy of treatment was assessed using
questions 3 and 4 from the International Index of Erectile Function (IIEF)
(4,5) at baseline (week 0) and at the end of 12 weeks of treatment. These
2 questions from the IIEF most closely address the definition of ED by
asking about the ability to achieve (Q3) and maintain (Q4) an erection.
Q3 and Q4 were scored on a scale from 1 (“almost never/never”)
to 5 (“almost always/always”) with 0 indicating “no attempt
at intercourse.” Efficacy was also assessed at 12 weeks using a global
efficacy question (GEQ) asking if treatment improved their erections.
Patients maintained an event log about sexual
stimulation and intercourse every time they took a dose of study drug
and/or engaged in sexual activity. The event log included the date of
medication/activity, whether study medication was taken, whether the subject
had sexual stimulation (“yes” or “no”), and whether
the subject had a successful sexual intercourse (responses: “yes”,
“no - erection did not last long enough,” “no - other reason”).
Their responses to these questions, including the proportion of successful
attempts at intercourse, were also used to assess efficacy.
Safety
Assessments
All observed or volunteered adverse events
that occurred during treatment or within 7 days of the end of treatment
were recorded. The investigator determined the severity of the adverse
event and whether it was related to drug treatment. Serious adverse events
were those that 1)- resulted in death, 2)- were life-threatening, 3)-
resulted in hospitalization or prolongation of existing hospitalization,
4)- resulted in a persistent or significant disability or incapacity,
or 5)- resulted in a congenital anomaly or birth defect. The safety analyses
included all patients who were randomized and took at least 1 dose of
study medication.
Data Analysis
Adverse events were tabulated by body system
and by severity (mild, moderate, or severe). Safety and efficacy data
were summarized using appropriate descriptive statistics. Descriptive
statistics were used to compare the 2 treatment groups for similarity
with respect to demographic and event log variables. The questions from
the IIEF were analyzed using univariate analysis of covariance methods.
The GEQ was analyzed using logistic regression. The data collected in
the event logs were analyzed in terms of the proportion of successful
attempts at sexual intercourse, which was estimated using an analysis
of covariance model. All tests of hypotheses were performed at the 5%
significance level (2-sided).
RESULTS
Patient
Demography
In 15 centers in Latin America (9 in Brazil
and 6 in Mexico), 263 patients were screened and 245 patients were randomized
to treatment (124 patients from Mexico and 121 patients from Brazil).
One hundred twenty-four patients were randomized to sildenafil and 121
to placebo. The 2 groups had similar demographics with mean ages of 55
and 58 years for the placebo and sildenafil treatment groups, respectively
(Table-1). Of the 245 patients who were randomized, 133 presented with
at least one concomitant or concurrent disease or syndrome (65 in the
placebo group and 68 in the sildenafil group). The most common concomitant
medical conditions were unspecified essential hypertension, diabetes mellitus,
and hyperplasia of the prostate (24%, 18%, and 7% vs. 29%, 24%, and 5%
for the placebo and sildenafil groups, respectively) (Table-1). The most
common concomitant medications were antihypertensives, insulin, and antidiabetic
drugs (Table-1).

The highest dose of sildenafil and placebo
was preferred by most of the patients. Of the 124 patients who started
treatment with sildenafil 50 mg, 4 (3%) were taking 25 mg, 48 (39%) were
taking 50 mg, and 71 (57%) were taking 100 mg at the end point of the
study (last dose dispensed).
Efficacy
The 2 primary efficacy variables, ability
to achieve an erection and ability to maintain an erection (IIEF Q3 and
Q4), were both significantly greater in the sildenafil group (P < 0.001)
compared with the placebo group (Figure-1). The secondary efficacy variables
derived from the patient event logs and the GEQ supported the results
of the primary analysis. The number of patients who felt the study drug
had improved their erections (Figure-2A) and the mean proportion of successful
attempts at sexual intercourse during the last 4 weeks (Figure-2B) was
also significantly higher for patients receiving sildenafil (P < 0.001).


Safety
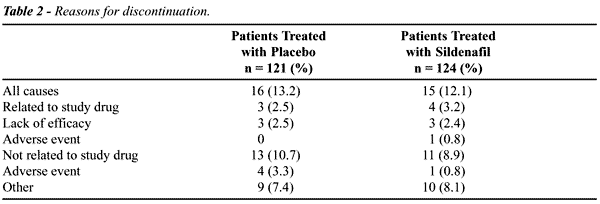
Fifteen (12%) of the patients receiving
sildenafil discontinued the study compared with 16 (13%) of the patients
receiving placebo (Table-2). Most of the discontinuations were not related
to the study drug. Of those related to the study drug, 3 patients in both
groups discontinued due to insufficient clinical response and 1 patient
receiving sildenafil discontinued due to an adverse event (severe transient
headache).
Forty-three (35%) of the patients receiving
sildenafil had treatment-emergent adverse events of all causalities, with
36 (29%) considered treatment-related by the investigators (Table-3).
This is contrasted with 24 (20%) of the patients receiving placebo reporting
treatment-emergent adverse events of all causalities, with 7 (6%) being
treatment-related. The most common all-causality adverse events were flushing
and headache, occurring in 0% and 5% for placebo versus 8.9% and 12.1%
for sildenafil, respectively. Most of the adverse events were considered
mild, with 5 in the sildenafil group and 4 in the placebo group considered
severe.

Ten patients (4 in the placebo group and
6 in the sildenafil group) had reductions in dosage due to adverse events
(Table-4). The most common adverse event leading to a reduction in dosage
was headache, which occurred in 2 patients in the placebo group and 4
patients in the sildenafil group. Six subjects (1 patient receiving sildenafil
and 5 patients receiving placebo) had serious adverse events, but these
were not considered to be related to the study medication (Table-5).


DISCUSSION
This
is the first study to systematically evaluate the effects of sildenafil
in Latin American men. The efficacy assessments demonstrate that sildenafil
was significantly more effective than placebo at improving the symptoms
of ED. Although the incidence of treatment-related adverse events was
higher for sildenafil than for placebo (29% versus 6%, respectively),
most of these (93%) were mild to moderate and only resulted in 1 discontinuation
due to a treatment-related adverse event in a sildenafil-treated patient.
Mild headache and flushing were the most frequently reported adverse events.
None of the 6 serious adverse events was considered to be related to treatment.
Thus, this study in 15 clinics in Brazil and Mexico demonstrates the safety,
tolerability, and effectiveness of sildenafil for the treatment of ED
in Latin American men.
An epidemiological study has examined the
prevalence and associated risk factors of ED in men in northeastern Brazil
(6), ranging from minimal to complete, was found in approximately 40%
of 602 men sampled. The prevalence of ED was significantly associated
with age, increasing from 28.5% for men between the ages of 40 and 44
years to 55.4% for men between the ages of 65 and 70 years. Other variables
that were significantly related to ED included self-reported diabetes,
depression, benign prostatic hyperplasia, caffeine consumption, and alcohol
use. The authors concluded that ED is a common health problem among Brazilian
men aged 40 to 70 years.
A Latin American epidemiological study conducted
in Colombia, Ecuador, and Venezuela surveyed 1946 men older than 40 years
of age (7). The authors reported prevalence of 19.8% and 33% for moderate-to-complete
and minimal ED, respectively. Health-related risk factors associated with
ED were hypertension, prostatic hyperplasia, diabetes, and associated
medication. Thus, both epidemiological surveys confirm the need for an
effective and well-tolerated treatment for ED in Latin American men. These
studies also confirm that this studied population was representative of
the general population in terms of concomitant medical conditions (e.g.,
diabetes and hypertension).
The results of this study are in agreement
with previous clinical trials of sildenafil. Similarly reported that the
highest dose of both sildenafil and placebo was preferred by most of the
patients (74% for sildenafil and 95% for placebo) (1). This agrees quite
well with the result in this study of 57% for sildenafil and 89% for placebo.
Similarly, in previous clinical trials, (1,2) the most common adverse
events that were considered treatment-related were headache, flushing,
and dyspepsia, which is in agreement with the results reported here. In
conclusion, this flexible-dose study suggests that oral sildenafil has
similar efficacy and safety in Latin American men as in North American
and European populations.
___________________________________
The research was supported by Pfizer Inc.
REFERENCES
- Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA: Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med, 338: 1397-1404, 1998.
- Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH: Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res, 10: 69-74, 1998.
- Aytac IA, McKinlay JB, Krane RJ: The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. Br J Urol Int, 84: 50-56, 1999.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A: The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology, 49: 822-830, 1997.
- Cappelleri JC, Rosen RC, Smith MD, Quirk F, Maytom MC, Mishra A: Some developments on the International Index of Erectile Function (IIEF). Drug Info J, 33: 179-190, 1999.
- Moreira ED, Lisboa LCF, Dale G: A cross-sectional population-based study of erectile dysfunction epidemiology in northeastern Brazil. J Urol, 163: 15 (Abst. # 65), 2000.
- Morillo LE, Esteves E, Costa A, Mendez H, Davila H. Medero N: The prevalence and associated risk factors of erectile dysfunction in men in Colombia, Ecuador, and Venezuela. Int J Impot Res (In press).
_____________________
Received: August 7, 2000
Accepted after revision: March 21, 2001
_______________________
Correspondence address:
Dr. Sidney Glina
Hospital Albert Einstein
Rua Almirante Pereira Guimarães, 360
São Paulo, SP, Brazil
Fax: + + (55)
(11) 3871-2466
E-mail: glinas@originet.com.br