ENURESIS
IN CHILDREN
(
Download pdf )
KELM HJÄLMÅS
Section of Pediatric Urology, Göteborg University, Göteborg, Sweden
ABSTRACT
Recent
demographic studies report a prevalence of nocturnal enuresis in at least
5 - 10% of six to seven year old children, most often boys, and in 0.5%
of the adult population. Bedwetting is the most common chronic problem
in childhood next to allergic disorders.
Nocturnal enuresis is still perceived as
a shameful condition and kept as a secret. But there is nothing shameful
about bedwetting. It is caused by a delay in maturation of the somatic
mechanisms responsible for sleeping dry all night. This delay is most
often hereditary in nature. With few exceptions, nocturnal enuresis is
not caused by psychosocial factors; but it generates psychological problems
for the child, especially evident as a deterioration of self-esteem.
Nocturnal enuresis results from nocturnal
polyuria and/or reduced bladder capacity and, in addition, the child’s
inability to wake up as a response to an over-full bladder.
With this background, the treatment for
nocturnal enuresis is based on enuresis alarm, which is meant to induce
arousal, and/or desmopressin (Minirin®, DDAVP) which reduces the amount
of urine produced. The alarm may be the first choice but needs a strong
support from both the prescribing physician and the parents. Desmopressin
is used when the alarm fails or is not accepted by the family and it is
increasingly becoming the first choice. Desmopressin in long-term treatment
may, like the alarm, give lasting cure of nocturnal enuresis, in particular
if medication is preceded with some weeks with bladder training (urotherapy).
Thus, the advice to the medical profession is to identify nocturnal enuresis
and prescribe treatment when the patient wants to sleep dry.
Key words:
enuresis; urinary incontinence; urination disorders; voiding dysfunction
Braz J Urol, 28: 232-249, 2002
INTRODUCTION
Introductory
Note on Terminology
The term “enuresis” in this text
is synonymous with “nocturnal enuresis” according to the recommendations
issued by ICCS (International Children’s Continence Society) (1).
Nocturnal enuresis (NE) or bedwetting, is the only kind of childhood urinary
incontinence that takes place as a complete incontinent micturition. All
other kinds of urinary leakage in children, whether daytime only or both
day and night should simply be denoted “urinary incontinence”
(UI).
The medical profession has not regarded
bedwetting as an exciting disorder. It is non-fatal and has a good prognosis.
For those families who ask for help, some will meet physicians who share
the traditional view on nocturnal enuresis as a marginal problem, requiring
little attention and no treatment. The standard answer from a physician
to a family searching help for their bedwetting son has often been “he
will grow out of it”. But this is not true for at least 5 per cent
of children with nocturnal enuresis (NE) who will remain enuretic for
the rest of their lives. In addition, recent investigations have shown
that NE is a very significant handicap for the affected child. Nocturnal
enuresis is still perceived as a shameful condition and kept as a secret.
But there is nothing shameful about bedwetting. As will be shown in the
following, NE is caused by a delay in maturation of the somatic mechanisms
responsible for sleeping dry all night. This delay is most often hereditary
in nature. With few exceptions, nocturnal enuresis is not caused by psychosocial
factors; but it generates psychological problems for the child, especially
evident as a deterioration of self-esteem.
The typical bedwetter is a little boy who,
while asleep, is doing something wrong, but he does not know what, and
definitely does not know what to do about it. His self-esteem is gradually
broken down and he sees himself as an inferior person. This occurs at
an age when intact self-esteem is extremely important for an optimal personality
development. So there is no doubt that bedwetting requires the physician’s
attention. The doctor’s door should be open for enuretic children
and they should receive adequate therapy as soon as they are motivated
for treatment, usually at an age around 6 - 7 years.
A new window to an improved understanding
of NE was opened in 1985 when a Danish research group discovered a somatic
disorder, nocturnal polyuria, in a group of bedwetting children (2). The
nocturnal polyuria was due to absence of the normally occurring increase
of antidiuretic hormone (aVP, arginine vasopressin) in plasma during the
night. This finding was in accordance with previous, largely overlooked
studies by Poulton (3) and the Indian researcher Puri (4) who had shown
larger nocturnal diuresis, and lower nocturnal urinary concentrations
of antidiuretic hormone, respectively, in enuretic as compared to non-enuretic
children.
In addition, nocturnal bladder overactivity
has also been found a significant cause in at least one third of bedwetters
(5). Moreover, some kind of arousal defect (or premature activation of
the micturition reflex) must be operative to allow the voiding to take
place during sleep. The need to empty the bladder during the night is
quite common in childhood, since nocturia (waking up from sleep to void)
is even more prevalent in school children than bedwetting (6).
DEFINITIONS
Nocturnal
Enuresis
Enuresis is defined as a complete or near-complete
micturition in the bed during sleep. The most common form of bedwetting
is monosymptomatic nocturnal enuresis (MNE) meaning that there are no
daytime symptoms pointing to bladder dysfunction. Thus, the child has
no pronounced urgency, no very frequent nor infrequent voidings and, most
important, no daytime incontinence. MNE is usually not a great problem
for children under the age of 5 years (7). Most children with MNE have
primary enuresis; i.e. there has never been a dry period of at least 6
months, in which case the enuresis is said to be secondary. This review
deals primarily with primary monosymptomatic nocturnal enuresis (PMNE).
The number of wet nights required in order
to regard nocturnal enuresis as a clinical problem is now generally considered
to be between 1 and 3 per month, because this is the threshold for most
affected children to be concerned and thus for most parents to seek help
(8).
Incontinence
versus Enuresis
All forms of wetting other than enuresis,
isolated bedwetting, should be categorized as incontinence, i.e. the loss
of small amounts of urine, never a complete void. The distinction between
the terms enuresis and incontinence has been found necessary for scientific
as well as clinical reasons. From the scientific viewpoint, monosymptomatic
nocturnal enuresis is a well-circumscribed entity and should not be mixed
up in research with other urine-losing conditions such as combined night-
and daytime incontinence. Research on such a mixed bag of conditions has
been common in the past resulting in studies lacking in scientific validity.
From the clinical viewpoint, traditional wisdom tells that the term enuresis
denotes an essentially innocent condition because everybody knows that
most enuretic children become dry as time goes by. “Enuresis”
became almost synonymous with urinary incontinence in childhood. Therefore,
many children with urinary incontinence due to organic conditions in the
nervous system or the lower urinary tract have been labeled “enuretics”
by less careful clinicians and sent home with a “wait and see”
message instead of getting immediate diagnostic attention. This has caused
unnecessary delay and sometimes even a worsening of prognosis for the
individual child. The more somber term incontinence makes the clinician
more attentive so time has now come to stop using the word “enuresis”
for every wetting child.
Outcome
of Treatment of Nocturnal Enuresis
The normal annual resolution rate of MNE
(15 to 17% per year) (9) should be accounted for when cure rates are reported.
The outcome of drug treatment for MNE is expressed as either full or partial
response while on the medication. Full response is defined as a reduction
in wet nights of more than 90% while partial response is defined as a
reduction in the number of wet nights of between 50% and 90%. Less than
50% reduction in wet nights is considered to be non-response. A lasting
cure is defined as a full response still present 6 months or longer after
discontinuation of pharmacotherapy (1). It is obvious that these definitions
of response require that the child’s nighttime wetting is carefully
recorded during at least 2 weeks before treatment. The 90% cut-off point
has been chosen in order to allow for the occasional wetting that can
occur up to 2 years after otherwise successful treatment during a night
when the child is running a fever or sleeps very deeply after a tiring
day.
In all studies on outcome of treatment of
MNE, it should be reported whether nighttime wetting was replaced with
nocturia (1).
Bladder
Capacity
The important variable is functional bladder
capacity (FBC) which is defined as the volume in the bladder when the
individual feels a genuine desire to void (DV). This is why it is difficult
in children to decide functional bladder capacity. Before 4 to 5 years
of age, or in older children with bladder dysfunction, the child is uncertain
about how to interpret signals from the bladder. A great up to 10-fold
variation of voided volumes is found even in older children with normal
bladders when the voidings are not supervised. “The normal child
voids when it is convenient, not always when the bladder is full”
(6). When we try to decide the FBC of a child in a hospital setting, a
common source of error is the child’s wish to please the investigator
(and to shorten the time spent in the laboratory) by performing several
small voidings with volumes well below the true bladder capacity. It is
therefore important to supervise the child, ask repeatedly “are you
sure that you need to void right now, or can you wait a little longer?”,
and allow voidings only when the child feels a genuine desire to void.
Because it is problematic to define a child’s
functional bladder capacity in the laboratory, the variable may be better
defined operationally. This is done by letting the family fill in a voiding
diary for at least a couple of days and define FBC as the largest measured
voiding excluding the morning voiding. The latter is a measure of the
nocturnal FBC after a dry night and is larger than any daytime micturition
volume (6). In enuretic children, nocturnal FBC can be measured at home
by weighing diapers.
With FBC defined in this way, it will be
found that normal daytime FBC for age complies well with the simple formula:
voided volume (ml) = [30 x (age in years + 1)]
meaning that the newborn baby has a bladder capacity around 30 ml and that the capacity increases with 30 ml per year until adult capacity is reached at age 15. Nocturnal capacity in a non-enuretic child (i.e., the volume of the first morning void) will be larger. The daytime FBC is significantly reduced when it does not reach more than 65 per cent of the formula value (10).
ETIOLOGY
Genetics
Nocturnal enuresis is a hereditary disorder.
Since long, it has been observed that bedwetting often occurred in several
members of the same family that has since been confirmed in an often cited
twin study (11). The mode of inheritance is autosomal dominant so if both
parents were enuretic as children, the risk for their offspring is 77%,
while if only one parent had NE, the risk is about 45%. Sporadic bedwetting
with no affected relatives occur in a little more of 30% of enuretic children,
but this figure may include polygenic or autosomal dominant inheritance
with low penetrance.
An interesting development is that genetic
aberrations leading to nocturnal enuresis are now becoming identified
with molecular genetic methods. Linkage analysis has shown foci on chromosomes
13 (12), 12 (13), 8, and 22 (14). This is however only the beginning of
the process of genetic mapping of NE. With increasing knowledge, a picture
of pronounced heterogeneity of both genotype and phenotype is emanating
(15), so the etiology of NE is characterized by a complex interaction
of genetic and environmental factors.
EPIDEMIOLOGY
Children
usually become dry by day before they become dry during sleep. The prevalence
of nocturnal enuresis has now been studied in many populations all over
the world (Table-1) (16-22). Only one study is longitudinal (16) while
all the rest are cross-sectional studies. In the French study by Lottmann
(21), the severity and consequences of enuresis were studied in a sub-sample
of 228 children (out of the 349 who had reported enuresis). In the sub-sample,
66% had more than one wet night per month, 37% more than one wet night
per week, and 22% wet the bed every night. Regarding consequences, 42%
of the 228 were “bothered a lot” while 15% were “not bothered
at all” by their enuresis. In contrast, 92% of the 228 mothers declared
that the enuresis had no effect on family life or the child’s behavior
at school. Fourteen per cent of mothers punished their child and only
13% intended to seek treatment for their child.
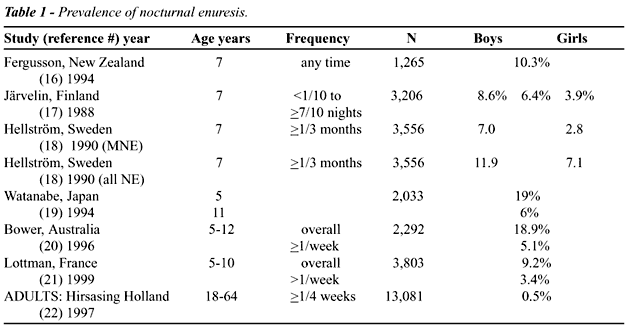
As is evident from the table, the figures
are not easy to compare because different selection criteria have been
used, especially regarding age and frequency of enuresis. Also, only one
study (18) reports on monosymptomatic enuresis while the others include
all patients with bed-wetting, hence even non-monosymptomatic patients
with NE and additional daytime symptoms (urgency, frequency, some also
day incontinence).
In early childhood, NE is more common in
boys than in girls but this gender difference disappears before adolescence.
Prevalence of NE at age 7 is significant since many children then start
school meaning more exposure to the environment and thus a greater awareness
of the problem. As a general rule it can be said that between 5% and 10%
of all 7-year-old children wet their beds often enough to be motivated
to receive active treatment. This makes NE, next to allergic conditions,
the most common chronic health problem in children in Western countries.
Recently the first reliable study on the
prevalence of NE in otherwise healthy adults has been published (22) (Table-1).
It should be noted that a prevalence of 0.5% in adults (with half of them
having primary NE) and 5% in children means that no less than 5% of children
with NE are at risk for life-long enuresis if they are not treated successfully
during the childhood years.
PATHOPHYSIOLOGY
Why
do some children wet their beds during sleep? The remarkable fact is that
the great majority of children sleep dry for 8 - 9 hours or more while
sometimes finding it hard to wait for only a couple of hours during daytime.
Thus, nocturnal dryness requires functions that are not present during
daytime. These are (i) reduction of nocturnal urine production so that
it does not exceed bladder capacity; and/or (ii) that the bladder detrusor
muscle is efficiently inhibited and relaxed; and (iii) that the sleeping
child is awakened by a full bladder, alternatively that the micturition
reflex is well inhibited so that the child is allowed sufficient time
to wake up before micturition ensues.
Thus, the basic pathophysiology of NE is
simple in that the bladder gets filled to capacity during sleep and needs
to be emptied. There are two main factors, working singly or in combination,
causing the bladder to become full. One is nocturnal polyuria because
urine production is not reduced during sleep as in the normal case. The
other factor is reduced nocturnal bladder capacity. The full bladder needs
to be emptied and then the important question is: does the child wake
up? If he wakes up, he walks to the bathroom and performs the socially
acceptable act of nocturia. If he does not wake up, the socially unacceptable
bedwetting ensues. Nocturia and enuresis share the same pathophysiological
background, a mismatch between diuresis and available bladder storage
space, with arousal or lack of arousal as the key difference.
Nocturia is even more common than NE in
children. Of healthy school-children 7 - 15 years of age, 35.2% reported
occasional nocturia, 3.6% nocturia at least once a week and 4.1% habitual
(every night) nocturia (23). With the addition of the 5 - 10% of children
who were nocturnal enuretics (7.9% in the cited study) (23), night-time
micturitions, asleep or after waking up, seem to occur in around 50% of
otherwise healthy school children.
Nocturnal
Polyuria
Normal subjects have a marked circadian
variation in urine output leading to a significant reduction of urine
excretion and a corresponding increase of urine osmolarity during sleep
(24). Decrease of renal urine production during the night allows for sleep
not disturbed by a full bladder. The circadian variation is present in
normal subjects regardless of age and has been attributed to nocturnal
increase of antidiuretic hormone (plasma vasopressin) (25) which is, however,
true only in childhood. In adolescence and adult age, the reduction of
nocturnal urine production occurs mainly due to a decrease in urinary
sodium excretion (24,26,30).
Relative nocturnal polyuria has been operationally
defined as a day/night urine ratio of < 1 which has been shown to exist
in around two thirds of children with PMNE. As mentioned previously, a
Danish research group in Aarhus looked at nocturnal urine production and
plasma vasopressin in children with NE and found a virtual absence of
day/night variation of vasopressin accompanied by nocturnal polyuria (2,27).
Thus, for the first time, a coherent physiological explanation for NE,
or at least a large part of the NE population, had been presented. The
new findings generated quite intense research resulting in, as expected,
both validating (28) and conflicting (29) data. This conflict has been
subsequently resolved by the mentioned finding that lack of nocturnal
increase in vasopressin ceases to be operative for the nocturnal polyuria
in enuretics at the beginning of adolescence (when it seems to be due
to nocturnal natriuresis). Presently, there is a consensus that relative
nocturnal polyuria is an important pathogenetic factor in around two thirds
of MNE patients regardless of age (those are the patients responding to
desmopressin, DDAVP® or Minirin®) while the remaining third has
inadequate nocturnal bladder storage.
Bladder Dysfunction in Nocturnal Enuresis
Previously, bladder function was thought
to be normal in enuretic patients. Recently, however, evidence about the
pathophysiological role of the bladder for NE has accumulated so it can
safely be said that the Bladder is Back in Business in NE (5,31-33). As
many as one third of all enuretic children, or even more, have a nocturnal
detrusor overactivity that will need specific treatment in order for the
enuresis to resolve. Especially non-polyuric bed-wetters, those who do
not respond well to desmopressin, should be suspected to have a malfunctioning
bladder with reduced capacity (34). Even children believed to have monosymptomatic
enuresis, that is no daytime symptoms, may have an overactive bladder.
Firstly, the bladder may be overactive only during sleep. Secondly, experience
tells that history taking is notoriously difficult in enuretic children
so that a negative history does not always exclude day symptoms with absolute
certainty.
Detrusor overactivity is revealed as pressure
peaks during cystometry. How does this finding translate into an inadequate
storage function of the bladder? The relevant fact is that an overactive
detrusor is not properly relaxed. Since the bladder is a muscle bag, it
cannot make use of its “true” capacity without a well functioning
inhibition of spontaneous detrusor activity during the filling phase.
In this context, it is interesting to note
that there is an association between childhood NE and adult detrusor overactivity.
In a retrospective study of 1000 urodynamic case records, 10% of the male
subjects were found to have idiopathic detrusor overactivity. Of these,
63% had suffered from childhood bedwetting (35). Corresponding figures
for females were 29% with bladder overactivity of whom 38% had been nocturnal
enuretics, which probably reflects the gender difference in childhood
bedwetting.
Convincing data on the role of daytime and/or
nighttime bladder dysfunction in NE have recently been published (5).
Forty-one children (33 boys and 8 girls), mean age of 10.4 years, with
PNE (3 or more wet nights weekly) had resisted previous treatment attempts
with desmopressin with or without an enuresis alarm. The enuresis was
considered to be monosymptomatic. All children were studied with daytime
cystometry, continuous natural fill cystometry and electroencephalography
during sleep, and recording of daytime and nighttime urinary output. Almost
none of the patients had nocturnal polyuria. All had a functional bladder
capacity smaller than expected for age. All 41 children were found to
have abnormal bladder function during sleep (detrusor overactivity or
frequent high-pressure small voidings) while 18 (44%) had normal urodynamics
at daytime. Thus, this study provides strong evidence that bladder dysfunction
is an important pathogenetic factor for NE, especially in children resistant
to conventional treatment with desmopressin and/or alarm. Also, that several
children with “monosymptomatic” NE, that is a normal bladder
during the day, may have nocturnal bladder dysfunction as a cause for
their enuresis.
Sleep
and Arousal
Relative nocturnal polyuria and/or reduction
of the nocturnal bladder capacity due to an overactive bladder cannot
explain why the enuretic child does not wake up to the sensation of a
full or contracting bladder so that the shameful enuresis could be transformed
into the acceptable act of nocturia. This is certainly something the enuretic
child himself would like to happen.
Sleep and arousal remain the least understood
factors in the pathophysiology of enuresis. Countless numbers of parents
have told physicians that their enuretic child is very difficult to arouse
(36) or rather, as the parents put it, “sleeps very deeply”.
Until recent years, medical research has been largely unsuccessful in
confirming this opinion of parents, not least because research on sleep
and arousal is extremely difficult. However, we have to question our scientific
methodology before drawing conclusions that conflict with what the parents
tell us. And today some modern studies seem to support the parent’s
view about abnormal sleep and arousal in enuretic children. By using auditory
signals (37), computerized EEG analysis (38), or inquiries (39), a defect
in arousal seems to be confirmed. Sophisticated EEG energy analysis has
indicated both greater depth of sleep and impaired arousal in enuretics
(40). Another recent study shows that the locus coeruleus, one of the
brain areas most responsible for arousal, is activated by bladder distension
only when the patient is in deep sleep, not in light sleep (41). This
finding agrees well with the results of EEG overnight monitoring in Yeung’s
enuretic children where EEG either did not show any change at the enuretic
event or a change from deep to lighter sleep with the enuresis occurring
in an aroused state but without actual full awakening (5).
Parent’s opinion about abnormal sleep
and arousal in children with NE are thus gradually confirmed. It should
be added, however, that even a child with perfectly normal sleep and arousal
may experience NE if there is an inadequate inhibition of the micturition
reflex due to an impaired processing of inhibitory signals in the brain
stem (42).
NE Pathophysiology
According to Watanabe
Nocturnal polyuria, arousal disorders and
detrusor overactivity have been integrated in a classification system
for NE proposed by Watanabe & Azuma (43). Based on overnight simultaneous
monitoring of electroencephalography and cystometry in several hundred
enuretic children, three main types of NE have been identified. Type I
is the most common (57% of patients) and regarded as an isolated mild
arousal disorder. Type II a (9%) shows an EEG that does not seem to respond
at all to a full bladder, thus an overt arousal defect, while in Type
II b (34%) there is, in addition to an arousal defect, continuous detrusor
overactivity in the cystometry during sleep.
OTHER CAUSES OF ENURESIS
Upper
Airway Obstruction
Surgeons have sometimes experienced that
NE resolves after the child had large adenoids or tonsils removed. One
study reports significant decrease or complete cure of NE in 87 (76%)
of 115 enuretic children (of whom 103 with primary NE) after surgical
removal of upper airway obstruction (44). The pathophysiology here is
not clear. Disturbed sleep may be a plausible explanation.
Constipation
Constipation may cause secondary NE or make
primary NE persist (33,45). A hypothetical explanation is that fecal retention
in the sigmoid colon and rectum exerts pressure on the bladder thus reducing
the storage capacity. The important implication is that constipation has
to be identified and treated in every child with NE.
Diabetes
mellitus and Insipidus
The polyuria in these conditions increases
the risk for NE, which is most often of the secondary type.
Minor
Neurological Dysfunction and ADHD
Children with minor neurological dysfunction
are more prone to NE, particularly if belonging to a lower social class
(46). Children with attention deficit hyperactivity disorders (ADHD) are
2.7 times more likely to have enuresis than the general child population
(47). The combination of ADHD and NE constitutes one of the rare indications
for treatment of NE with tricyclic antidepressants.
Sexual
Abuse
We have become aware that sexual abuse must
count among factors that may lead to NE (most often secondary and non-monosymptomatic).
A strong suspicion would prompt full investigation (48).
Non-monosymptomatic
Enuresis
Although this review deals primarily with
primary monosymptomatic nocturnal enuresis (PMNE), it should be added
that children with urinary tract infection, infravesical obstruction,
neurogenic bladder, serious psychiatric disorders, and other conditions
may be wetting their beds. Their nocturnal incontinence is, however, with
very few exceptions combined with daytime symptoms, in particular day
wetting. One possible exception is congenital infravesical obstruction
in boys (posterior urethral valves) who sometimes present with primary
NE without daytime symptoms. It is, however, wise to remember that PMNE
with isolated bedwetting as the only symptom is a well circumscribed condition
that should be identified when present, thus avoiding clinical confusion
generated by the huge number of childhood disorders that may have bedwetting
as one of its symptoms.
PSYCHOLOGICAL ASPECTS
Fortunately,
it is now long since PNE was looked upon as a disorder of the mind. “Pediatricians
should treat PNE as a common biobehavioral problem without a psychiatric
component” (49). While it seems clear that psychopathology is not,
with few exceptions, the cause of PNE, research has lately been focused
on the sometimes serious psychological consequences caused by enuresis.
Several recent studies have been unanimous in reporting that PNE generates
substantial feelings of shame and inferiority in the enuretic child, in
particular evident as depression of the child’s self-esteem and self-image
(17,50-51). There is a small but significant risk for psychiatric disorders
and problems with social adjustment in enuretic children beyond the age
of 10 years (17). This circumstance certainly constitutes a strong indication
for starting active treatment as soon as the child is ready to receive
it, especially since it has been shown that the child’s self-esteem
becomes normal within 6 months after successful treatment (51).
Most parents feel tolerant towards their
enuretic child with the understanding that the child cannot control the
problem. However, up to one third of parents is less understanding and
intolerant, and they may even punish their child (52). Parental intolerance
is strong predictor that any attempts to treat the enuresis will fail.
INVESTIGATION
For the management of a child with NE, the most important diagnostic procedure is to identify monosymptomatic enuresis by history. Once the history has classified the child as monosymptomatic by the exclusion of pronounced urgency, frequency or infrequent voidings, and in particular daywetting, only minimal additional diagnostic work is needed.
History
Pediatric history taking is never easy and
the enuretic child is certainly not an exception. Most of the history
is filtered through the parents who often tend to give answers they believe
to be the right answers, not necessarily the correct answers. Also, the
references used by the parents are the child’s siblings and friends.
If there happens to be a high prevalence of urgency and frequency among
these children, the parents may look upon their 7-year-old son’s
speedy and frequent rushes to the toilet as normal behavior. Also, NE
is still looked upon as a shameful condition by many parents and children
alike which will add bias to the history taking. Finally, history taking
often involves teaching parents and children to understand the actual
meaning of the concepts of urgency and frequency.
Urgency
Urgent desire to void is present in no less
than 22% (imperative urgency in 16%) of healthy 7-year-old schoolchildren
in Sweden (18). What the physician needs to know is whether the child
has pronounced urgency with last-minute races to the bathroom threatening
to produce urge incontinence. It is also of value to find out if the urgency
is due to holding the urine to the last minute (so called voiding postponement)
or to a sudden imperative detrusor contraction. The voiding postponers
are relatively easy to identify because they are intensely occupied in
play while giving bodily signals that they feel a genuine desire to void,
such as crossing their legs and wriggling while sitting.
Frequency
and Infrequent Voiding
The normal range in 7-year-olds is 3 to
7 micturitions daily (18). Detrusor overactivity (unstable bladder) leads
to eight or more voidings a day. But it is equally important to recognize
infrequent voiding with three or less voidings due to detrusor underactivity.
The latter is most often caused by bladder distension as a sequel of long-standing
detrusor-sphincter dyscoordination, which is a sign of serious bladder-sphincter
dysfunction.
Daywetting
This is the most important symptom to exclude
in order to classify the enuresis as monosymptomatic but it is also quite
often the most difficult to elucidate, due to the parent’s and the
patient’s understandable tendency to subdue information which they
are ashamed of. However, the history has to penetrate this question carefully.
“Wetting” may be denied while a question about “dampness”
may receive a positive response. If there is any amount of daytime incontinence
present, the enuresis is definitely not monosymptomatic. The child’s
daytime and nighttime incontinence is most probably caused by detrusor
overactivity and will need specific investigation and therapy.
Symptoms
Pointing to Bladder Emptying Problems
Difficulty to empty the bladder points to
bladder-sphincter dysfunction or organic, anatomical or neurogenic, disorders
of the lower urinary tract and is present in 1% of an unselected population
of 7-year-olds in Sweden (18). Such conditions, if present, do not allow
the child’s bedwetting to be classified as monosymptomatic. The child
may experience that it is difficult to start the voiding or has to strain
with the abdominal muscles or press with the hand against the suprapubic
area during voiding. The urine stream may be weak and can be labeled “non-competitive”
in a boy voiding together with friends. Finally, a healthy child always
empties the bladder in one portion. A micturition divided in several discrete
portions is a sure sign of an underactive detrusor and/or infravesical
obstruction of whatever cause.
Voiding
Diary
The voiding diary is included here in the
History section because the diary supplements the history in an invaluable
way. When first asked about frequency of daytime voidings or the number
of enuretic events per week, most parents cannot give a reliable answer.
Time is saved if the parents can receive the diary by mail before the
first office visit and bring it duly filled in at the first visit. The
voiding diary should be maintained for at least one week and will give
a clear picture of the child’s micturition pattern including any
daytime wetting or “dampness” and the number of wet nights.
The parents should be asked to observe whether there is more than one
enuretic event during a wet night. A baseline is thus established to be
compared with the results of subsequent therapy.
Diet
The pattern of food and fluid intake during
an ordinary day has to be looked into. It is quite common to find that
the child takes massive amounts of soft drinks just before going to bed,
a habit which in itself can lead to enuresis.
Emotional
Impact
One of the physician’s first questions
to the child should be “Do you know why you visit me today?”
or even “Is there anybody in this room wanting to stop wetting the
bed?”. Even if a shy child does not give a verbal response, the child’s
body language may tell a lot about the perceived impact of the enuresis.
Children who do not seem to bother about these questions probably do not
bother much about their enuresis either, so they may not yet be motivated
to receive treatment. Most often, however, enuretic children clearly react
in a distressful way to the questions and some even say in plain language
that they would very much like to get rid of their bedwetting. Since it
is important to assess the emotional impact on the child, questions to
child and parents should follow whether there has been any teasing from
family and schoolmates and if the child avoids sleeping over in a friend’s
home or participate in school trips. It is important to tell the child,
at this stage, that NE is a very common condition. The enuretic child
feels very much alone with his problem which he and all other affected
children keep as a shameful secret. The child feels enormous relief when
understanding that he is not alone, after all.
Primary
or Secondary Enuresis?
The history has to include this question
which is not, however, of any great consequence. Secondary enuresis presenting
before age 4 to 5 years as a rule has the same characteristics as primary
enuresis. When presenting later, secondary enuresis may be due in some
children to psychological trauma or urinary tract infection. It is then
seldom monosymptomatic NE but rather nighttime and daytime incontinence.
Physical
examination
An ordinary physical examination should
be performed at the first office visit. In order to exclude neuropathy,
the lower back, legs and feet should be inspected and tendon reflexes
tested. Genitals should be examined since the only part of the urinary
tract visible to the naked eye is the urethral meatus. A rectal exploration
should be performed in order to check the tonus of the anal sphincter
and exclude fecal retention in the rectum.
Urinalysis
A dip stick will exclude protein, glucos,
hematuria and most urinary pathogens. If there is a history of previous
UTI, urinary culture should be added.
Other
Investigations
If the history has clearly identified monosymptomatic
NE there is presently no indication for additional investigations. This
situation may change in the future since the mode of management will depend
on the relative importance of nocturnal polyuria, bladder dysfunction,
and arousal disorder, for the pathogenesis of the individual child’s
enuresis. Some specialized enuresis centers have already started to assess
nocturnal urine production by weighing diapers, and bladder function by
measurement of urinary flow and post-void residual. For the evaluation
of arousal there is as yet no clinically useful test available. However,
most physicians taking care of enuretic children still use an ex juvantibus
approach to this diagnostic question. For example, polyuria is probably
an important factor and bladder dysfunction less important for the child
who responds well to desmopressin, and vice versa.
Ultrasound of kidneys and bladder is an
optional examination that is quite often used but very seldom gives any
information in a child with monosymptomatic NE. The situation is quite
different, of course, if the child has combined daytime and nighttime
incontinence in which case a full neuro-urological investigation is always
indicated.
TREATMENT
Management
of NE is based on 4 principles:
a)- Verify the child’s motivation to be treated and exclude confounding
psychosocial factors;
b)- Information and instruction about daily habits underlining the importance
of having regular fluid intake and voidings and relaxed routines at bedtime;
c)- Enuresis alarm;
d)- Antidiuretic medication (desmopressin, DDAVP®, Minirin®).
This section will also cover other pharmacological
therapy (detrusor relaxing agents, tricyclic antidepressants, and prostaglandin
synthesis inhibitors), urotherapy, prevention of relapses, choice of treatment,
and how to handle non-responders.
Motivation
It is not uncommon for a 4 - 5 year old
bedwetter to be brought to the physician’s office because the parents
are concerned about the bedwetting while the child is not. NE often requires
a long course of treatment that may last for one or several years. It
is therefore important that the enuretic child is at least moderately
motivated to receive treatment and mature enough to understand that he/she
is expected to participate actively, and that it will take time to become
dry. The child’s motivation is checked with the simple question “Do
you want to become dry at night?”.
Confounding
Psycho-Social Factors
Broken homes, social misery, intolerant
parents and child behavioral problems should be identified. These factors
predict treatment failures.
Regulating
Daily Habits
Today, school children often delay most
of their eating and drinking until after school hours. Girls in particular
often avoid to visit the busy and sometimes unsafe and not-so-clean school
toilets. Consequently, many school children do not void at all between
the morning micturition and the time when they return back home from school.
The risk for bedwetting increases when the bladder has not been emptied
for 8 hours during daytime and then is exposed to increased urine production
during evening hours. Quite a few enuretic children stop wetting their
beds just by establishing a regular drinking and voiding schedule during
the day. Such a schedule will often need to be discussed with and supervised
by the child’s teacher and school nurse.
Enuresis
Alarm
The enuresis alarm is an effective way to
treat monosymptomatic NE although not quite as effective as described
in older literature. A well done meta-analysis reports lasting cure in
43% of patients (53). Bed mats and body-worn alarms are equally effective.
Alarm treatment is slow in the start so it should continue for at least
6 to 8 weeks before being considered ineffective. However, alarm treatment
requires that the parents participate actively especially in the initial
stages of therapy. Thus, compliance remains a problem with drop-out rates
seldom reported in the studies. In a study of 88 adults who had been treated
with enuresis alarm 10 to 20 years earlier, 3 had not ceased bedwetting
until age 20 to 36 and 4 were still having NE. Of the cured, 16 remembered
the alarm as awkward and embarrassing (54). Failure of alarm treatment
is predicted by lack of supervision during the treatment period, inconsistent
or incorrect use of the alarm, technical problems with the alarm or, most
often, that the child does not wake up when the alarm sounds (55). The
rest of the family usually does, verifying the arousal disorder in NE.
The mode of action of the alarm has been
believed to be an improvement of arousal when the bladder is full. This
may be true but lacks scientific proof. An interesting recent finding
is that the alarm increases the nocturnal bladder capacity in those children
who become dry. This may explain why children, after successful alarm
treatment, are often able to sleep dry with no nocturia (56).
A modern development of the alarm method
is by monitoring bladder volume during the night using a miniaturized
ultrasonic transducer which is carried on a belt over the suprapubic area
of the sleeping child. At a predetermined bladder volume, a sound signal
is emitted intending to wake the child before the enuresis occurs (57).
Desmopressin,
dDAVP
Placebo-controlled studies have shown that
the anti-diuretic drug desmopressin (dDAVP) is significantly more effective
against NE than placebo (58). Around 62% of patients become dry or reduce
the number of wet nights with at least 50% (59) which agrees well with
the 69% of enuretic children found by Poulton to have nocturnal polyuria.
In a long-term home-based study monitoring nocturnal urine production
and enuretic episodes, the responders to desmopressin treatment were those
with nocturnal polyuria (60). Relapse after short-term treatment is rather
the rule while long-term treatment may yield better cure rates (61). In
order to elucidate the effect of long-term desmopressin, a large multi-center
prospective study (the Swedish Enuresis Trial, SWEET) was performed (59),
comprising 393 children aged 6 - 12 years with monosymptomatic NE and
10 or more wet nights during 4 weeks. Intranasal desmopressin in titrated
dose 10 - 40 µg was given until at least a 50% reduction in the
number of wet nights occurred which happened for 245 (62%) of the children.
The 245 responders started a 1-year treatment period which resulted in
75 (31%) becoming completely dry while still on desmopressin and another
75 (31%) cured without medication. Most of the full responders became
dry during the first 6 months of treatment. An intention-to-treat analysis
thus showed lasting cure in 75 of the original 393 children, that is 23%
which is only marginally better than spontaneous resolution. The lesson
learnt from the SWEET study is that among children who had an initial
response of > 50% reduction of wet nights (and these are probably the
children with nocturnal polyuria as their main pathogenesis), 31% were
dry and continued to be dry after stopping desmopressin treatment.
Besides polyuria, predictors of response
to desmopressin are fewer wet nights (< 3 per week), only one enuretic
event per night, age 8 years or more, and a lasting response to a small
dose of desmopressin (20 µg intranasal or 0.2 mg per os). In addition,
daytime bladder capacity in the normal range (that is the capacity expected
for age), measured as the largest voiding in a 2 day voiding diary, predicts
good response to desmopressin (32,33). In contrast, morning urine osmolarity
or heredity for NE does not have any predictive value.
Side effects are moderate headache or abdominal
pain in 3% of patients (59), seldom severe enough to interrupt treatment.
Since desmopressin is a potent antidiuretic drug there are rare accounts
of severe water retention with hyponatremia and convulsions (62) but none
with a lethal outcome. The patient should not drink any fluids for 2 hours
before taking desmopressin.
Combined
Treatment with Alarm and Desmopressin
Enuresis alarm and desmopressin are not
antagonists. They are rather synergistic when used together, so a combined
treatment should be tried when monotherapy with alarm and desmopressin
has been unsuccessful. After around 6 weeks, desmopressin is discontinued
and alarm continued until NE is cured. The fast action of desmopressin
is believed to facilitate the child’s adaptation to the alarm. Compared
to monotherapy with desmopressin and alarm, the combination has been found
to be particularly effective for children with psychosocial problems (63).
Other Pharmacological Therapy
Detrusor
Relaxing Drugs
Detrusor overactivity, at least during nighttime,
is an important pathogenetic factor even for monosymptomatic NE (5), especially
in children who do not show a satisfactory response to alarm and/or desmopressin.
This condition can be diagnosed with overnight cystometry which is hardly
feasible in everyday clinical practice. It is permissible, therefore,
to try a detrusor relaxing drug ex juvantibus in addition to the ongoing
therapy with alarm and/or desmopressin. Oxybutynine is used in dosage
of 5-mg bid to children 7 - 10 years of age. A recent alternative is tolterodine
which in adults seems to have the same effect on the overactive detrusor
as oxybutynine but with fewer side effects such as dry mouth and blurred
vision. Tolterodine is not yet approved for use in children, but a recent
study in children 5 to 10 years of age with overactive bladder has shown
good effect of tolterodine with a virtual absence of side effects. The
dosage in children was 1-mg bid, which is half of the recommended dose
for adults (64). It should be noted that detrusor relaxing drugs given
as monotherapy are not efficient against NE. They have their place as
adjuncts to urotherapy (see below) and enuresis-specific therapy such
as alarm and/or desmopressin.
Tricyclic
Antidepressants
Imipramine and other members of the same
drug family are still widely used for treatment of enuresis. However,
they cannot be generally recommended for treatment of this non-fatal disorder
because of their potentially lethal side-effects with deaths reported
both in patients and their younger siblings (65). Also, the reported lasting
cure rate of only 17% (66) after imipramine therapy restricts the use
of these drugs. However, for a small group of very carefully selected
patients with NE, tricyclic antidepressants may be of value. Adolescent
boys with ADHD and persistent NE belong to this group. Given the adverse
effects, especially the cardiomyotoxicity, and the individual variability
in plasma levels, responsible use of such medication includes careful
monitoring by the prescribing clinician, preferably a child psychiatrist.
Inhibitors
of Prostaglandin Synthesis
As mentioned, nocturnal polyuria in adolescent
and adult patients with NE is as a rule caused by an increase in nocturnal
excretion of solutes, especially sodium (26). This may be the explanation
that cyclo-oxygenase inhibitors (such as diclofenac) which are known to
reduce urinary solute excretion were effective against NE in a double-blind
placebo-controlled trial (67). The future role of these drugs for treatment
of NE remains to be elucidated.
Urotherapy
(“Bladder Training”)
For enuretic children not responding to
either of alarm, desmopressin, or the combination, the bladder may be
the culprit even if historical data have allowed the enuresis to be classified
as monosymptomatic. For nonresponders to conventional treatment, detrusor
relaxing drugs should be considered, as already mentioned, but non-pharmacological
management with bladder-specific treatment, urotherapy, should always
be the first step. This is particularly true for children with border-line
urgency, frequency, or infrequent voiding. Urotherapy is cognitive training
which makes use of the fact that the normal bladder is under complete
cortical, voluntary control in the healthy individual. Urotherapy involves
information about what is normal and abnormal concerning the lower urinary
tract, instruction about regular habits as regards drinking, voiding and
sleeping, and a schedule with voidings at predetermined times. In addition,
the child is coached repeatedly that he or she will really become able
to take control of the bladder. This regimen is not magic but it puts
the onus of responsibility where it belongs, on the owner of the bladder,
and where it next belongs, on the parents. The schedule with voidings
at predetermined times is the most efficient part of the training program.
The child seems to be greatly impressed when he or she succeeds, for the
first time, to start a voiding at will without having felt a prior desire
to void: “I may be able to become Boss of my Bladder, after all”.
It has been shown in several studies that with these simple measures,
the symptoms of an overactive or underactive bladder will disappear in
65% to 75% of the patients.
Especially children with bladder distension
and infrequent voidings need a strict micturition regimen supervised by
a urotherapist in order to increase the number of regular voidings during
the day. Twenty-two children with therapy-resistant NE were initially
considered to be monosymptomatic although, when the history was carefully
revised, they were shown to have “lazy bladders” with infrequent
voidings. After having attained a normal number of daytime voidings, 20
of the 22 were cured of their bedwetting, either without further treatment
or with the help of desmopressin or alarm (68). Since bladder problems
are so difficult to exclude with history, a most useful option is to let
all enuretic children start treatment with a few weeks of urotherapy as
described, subsequently adding specific anti-enuretic therapy.
Prevention
of Relapse
Neither of the commonly used management
modalities for NE can claim to be really successful. Less than half of
affected children achieve permanent cure of their NE. New management strategies
are certainly needed. As a promising example, a structured withdrawal
program at the end of desmopressin or alarm treatment is reported to reduce
relapses substantially. A key factor here is said to be, in the psychologist’s
language, “internalization of the child’s success” (55).
These words seem to mean, in the child’s own language “I am
dry, not because I have been treated but because I am dry”.
Choice
of Treatment
A sensible recommendation for treatment
would require a thorough analysis of the relative importance of the different
pathogenetic factors and their causes in the individual child with NE,
that is nocturnal polyuria, bladder dysfunction, arousal disorder, and
possible psycho-social confounders. While this may be the ultimate goal
it is clearly not yet a practical suggestion. There are some predictors
available as mentioned above regarding desmopressin and alarm, i.e.
1)- alarm should not be used in families living under stress or where
there is parental intolerance towards the child;
2)- alarm should be preferred if the child has frequent NE (>3 per
week) or when there is a strong suspicion of reduced bladder capacity;
3)- desmopressin is the treatment of choice where nocturnal polyuria has
been established (which will need weighing of diapers).
But by and large our present knowledge does
not allow us to identify “the right treatment” for any child
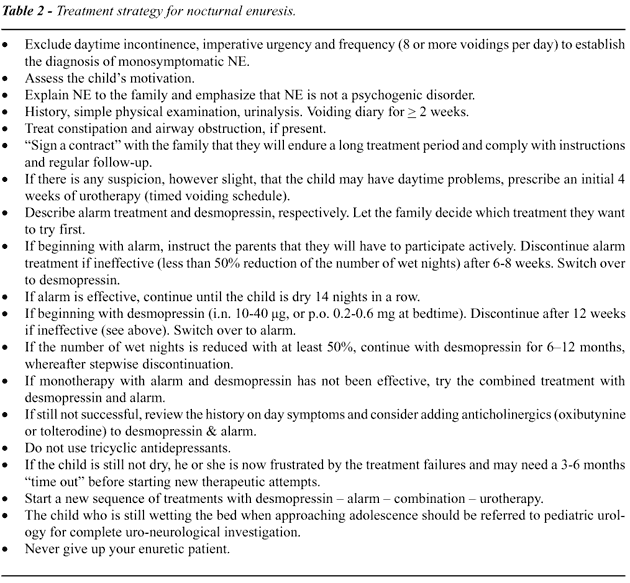
with NE. When the rationale is not there we will have to do with Treatment
Strategies of which a useful one is presented in Table-2. This strategy
proposes to let the child and the family choose mode of therapy themselves
after having received full information about the options (69). Whatever
first step is taken does not matter much because sequential switching
between different treatments will be the rule for children who do not
respond.
How to Handle Non-responders
Even with sequential treatment over and
over again some children do not get rid of their bedwetting. It then becomes
evident that the concern felt by the 7-year-old grows into an immense
burden for the enuretic adolescent ready to take the first steps into
an independent life. Among adult bedwetters the majority consider the
enuresis to be their most significant life problem (22) and many avoid
the opposite sex and stay unmarried in fear of revealing their shameful
secret. For the adolescent enuretic it is of huge importance that he is
not rejected by his physician but instead is met by an optimistic attitude
and always feels welcome to discuss other lines of action.
It is also important to remember that a
few patients have an organic cause for even monosymptomatic NE. After
one year or more of failed treatment attempts it is therefore imperative
to refer the patient to a full uro-neurological investigation including
cystometry, voiding urethrocystography and urethrocystoscopy in addition
to careful neurological examination.
FUTURE RESEARCH PRIORITIES
The
main priority for future clinical research is to try to find ways out
of the present rather gloomy situation regarding treatment. The honest
view is that we lack a really efficacious therapy for this very common
disorder. As always, this depends on a lack of understanding of the basic
mechanisms underlying the heterogeneous condition, or rather symptom,
of NE. If polyuria, poor arousal, and bladder dysfunction were the only
important pathogenetic factors in NE, how comes that we do not cure all
patients with NE? There is a large space available here for new and original
thinking around the etiology of enuresis.
Further studies are needed on the role of
urinary solute excretion and its regulation by hormones and prostaglandins,
and on treatments aiming at reducing nocturnal solute excretion and, thereby,
polyuria.
Hopefully, molecular genetic research will
be able to open new avenues by identifying the genes at fault and their
gene products. The gene product is an enzyme that will induce the synthesis
of a protein that may eventually turn out to be a neural transmitter involved
in the conversation between different centers in the pontine area: the
micturition center, the arousal center and the noradrenergic nucleus responsible
for the control of vasopressin output. Once we know a little more about
how the faulty genes express themselves in the phenotype we may be able
to help those enuretic children who are presently unresponsive to our
treatment attempts. Our ultimate goal must be to prevent nocturnal enuresis
to persist in adult age.
REFERENCES
- Nørgard JP, van Gool JD, Hjälmås K: Standardization and definitions in lower urinary tract dysfunction in children. Brit J Urol, 81: Suppl 3: 1-16, 1998.
- Nørgaard JP, Pedersen EB, Djurhuus JC: Diurnal anti-diuretic-hormone levels in enuretics. J Urol, 134: 1029-1031, 1985.
- Poulton EM: Relative nocturnal polyuria as a factor in enuresis. Lancet, 2: 906-907, 1952.
- Puri VN: Urinary levels of antidiuretic hormone in nocturnal enuresis. Indian Pediatr, 17: 675-676, 1980.
- Yeung CK, Chiu HN, Sit FK: Bladder dysfunction in children with refractory monosymptomatic primary nocturnal enuresis. J Urol, 162: 1049-1054, 1999.
- Mattsson S, Lindström S: Diuresis and voiding patterns in healthy school children. Brit J Urol, 76: 783-789, 1995.
- Bloom DA, Seeley WW, Ritchley ML: Toilet habits and continence in children: an opportunity sampling in search of normal parameters. J Urol, 149: 1087-1090, 1993.
- Butler RJ: Establishment of working definitions in nocturnal enuresis. Arch Dis Child, 66: 267-171, 1991.
- Forsythe WI, Redmond A: Enuresis and spontaneous cure rate - study of 1129 enuretics. Arch Dis Child, 49: 259-263, 1973.
- Hjälmås K: Micturition in infants and children with normal lower urinary tract. Scand J Urol Nephrol, 12: Suppl 37: 1-130, 1976.
- Bakwin H: Enuresis in twins. Arch Pediatr Adolesc Med, 121: 222-225, 1971.
- Eiberg H, Berendt I, Mohr J: Assignment of dominant inherited nocturnal enuresis (ENUR1) to chromosome 13q. Nat Genet, 10: 354-356, 1995.
- Arnell H, Hjälmås K, Jägervall M: The genetics of primary nocturnal enuresis: inheritance and suggestion of a second major gene on chromosome 12q. J Med Genet, 34: 360-365, 1997.
- Von Gontard A, Eiberg H, Hollman E: Molecular Genetics of Nocturnal Enuresis: Linkage to a Locus on Chromosome 22. In: Djurhuus JC, Hjälmås K, Jørgensen TM (eds.). Proceedings, Fourth International Workshop, International Research Center, Aarhus. Scand J Urol Nephrol, 33: (Suppl 202): 76-80, 1999.
- Vvon Gontard A, Eiberg H, Hollman E: Molecular genetics of nocturnal enuresis: clinical and genetic heterogeneity. Acta Paediatr, 87: 571-578, 1997.
- Fergusson DM, Horwood LJ: Nocturnal enuresis and behavioral problems in adolescence: a 15 year longitudinal study. Pediatrics, 94: 662-668, 1994.
- Järvelin MR, Vikeväinen-Tervonen L, Moilanen I: Enuresis in seven-year-old children. Acta Paediatr, 77: 148-153, 1988.
- Hellström A-L, Hanson E, Hansson S: Micturition habits and incontinence in 7-year-old Swedish school entrants. Eur J Pediatr, 149: 434-437, 1990.
- Watanabe H, Kawauchi A, Kitamori T: Treatment system for nocturnal enuresis according to an original classification system. Eur Urol, 25: 43-50, 1994.
- Bower WF, Moore KH, Shepherd RB: The epidemiology of childhood enuresis in Australia. Brit J Urol, 78: 602-606, 1996.
- Lottmann H: Enuresis treatment in France. Scand J Urol Nephrol, 33 ( Suppl 202): 66-69, 1999.
- Hirasing RA, van Leerdam FJ, Bolk-Bennink L: Enuresis nocturna in adults. Scand J Urol Nephrol, 31: 533-536, 1997.
- Mattsson S: Urinary incontinence and nocturia in healthy school children. Acta Pædiatr, 83: 950-954, 1994.
- Rittig S, Matthiesen TB, Hunsballe JM: Age-related changes in the circadian control of urine output. Scand J Urol Nephrol, 29 (Suppl 173): 71-74, 1995.
- George PLC, Messerli FH, Genest J: Diurnal variation of plasma vasopressin in man. J Clin Endocr Metab, 41: 332-338, 1975.
- Rittig S, Matthiesen TB, Pedersen EB: Sodium regulating hormones in enuresis. Scand J Urol Nephrol, 33 (Suppl 202): 45-46, 1999.
- Rittig S, Knudsen UB, Nørgaard JP: Abnormal diurnal rhythm of plasma vasopressin and urinary output in patients with enuresis. Am J Physiol, 256: F664-671, 1989.
- Aikawa T, Kasahara T, Uchiyama M: Circadian variation of plasma arginine vasopressin concentration or arginin vasopressin in enuresis. Scand J Urol Nephrol, 33 (Suppl 202): 47-49, 1999.
- Läckgren G, Neveus T, Stenberg A: Diurnal plasma vasopressin and urinary output in adolescents with monosymptomatic nocturnal enuresis. Acta Pediatr, 86: 385-390, 1997.
- Hunsballe JM, Hansen TK, Rittig S: The efficacy of DDAVP is related to the circadian rhythm of urine output in patients with persisting nocturnal enuresis. Clin Endocrinol, 49: 793-801, 1998.
- Kirk J, Rasmussen PV, Rittig S: Micturition habits and bladder capacity in normal children and in patients with desmopressin-resistant enuresis. Scand J Urol Nephrol, 29 (Suppl 173): 49-50, 1995.
- Rushton H, Belman AB, Zaontz M: The influence of small bladder capacity and other predictors on the response to desmopressin in the management of monosymptomatic nocturnal enuresis. J Urol, 156: 651-655, 1996.
- Eller DA, Homsy YL, Austin PF: Spot urine osmolality, age and bladder capacity as predictors of response to desmopressin in nocturnal enuresis. Scand J Urol Nephrol, 31(Suppl 183): 41-45, 1997.
- Nevéus T, Hetta J, Cnattingius S: Depth of sleep and sleep habits among enuretic and incontinent children. Acta Pediatr, 88: 748-752, 1999.
- Moore KH, Richmond DH, Parys BT: Sex distribution of adult idiopathic detrusor instability in relation to childhood bedwetting. Br J Urol, 68: 479-482, 1991.
- Wille S: Nocturnal enuresis: sleep disturbance and behavioural patterns. Acta Pediatr, 83: 772-774, 1994.
- Wolfish NM, Pivik RT, Busby KA: Elevated sleep arousal thresholds in enuretic boys: clinical implications. Acta Pediatr, 86: 381-384, 1997.
- Kawauchi A, Imada N, Tanaka Y: Changes in the structure of sleep spindles and delta waves on electroencephalography in patients with nocturnal enuresis. Br J Urol, 81(Suppl 3): 72-75.
- Nevéus T, Läckgren G, Stenberg A: Sleep and night-time behaviour of enuretics and non-enuretics. Br J Urol, 81 (Suppl 3): 67-71, 1998.
- Hunsballe JM: Sleep studies based on electroencephalogram energy analysis. Scand J Urol Nephrol, 33 (Suppl 202): 28-30, 1999.
- Watanabe H, Kawauchi A: Locus coeruleus function in enuresis. Scand J Urol Nephrol, 33: (Suppl 202): 14-17, 1999.
- Ornitz EM, Russel AT, Hanna GL: Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biol Psychiatry, 45: 1455-1466, 1999.
- Watanabe H, Azuma Y: A proposal for classification system of enuresis based on overnight simultaneous monitoring of electroencephalography and cystometry. Sleep, 12: 257-264, 1989.
- Weider DJ, Sateia MJ, West RP: Nocturnal enuresis in children with upper airway obstruction. Otolaryngol Head Neck Surg, 105: 427-432, 1991.
- Loening-Baucke V: Urinary incontinence and urinary tract infection and their resolution with treatment of chronic constipation of childhood. Pediatrics, 100: 228-232, 1997.
- Lunsing RJ, Hadders-Algra M, Touwen BC: Nocturnal enuresis and minor neurological dysfunction at 12 years: a follow-up study. Dev Med Child Neurol, 33: 439-445, 1993.
- Robson WLM, Jackson HP, Blackhurst D: Enuresis in children with attention deficit hyperactivity disorder. SMJ, 90: 503-505, 1997.
- Forbes FC: Children with enuresis. Nowadays, a strong suspicion of sexual abuse would prompt full investigation. BMJ, 316: 777, 1998.
- Friman PC, Handwerk ML, Swearer SM: Do children with primary nocturnal enuresis have clinically significant behavior problems? Arch Pediatr Adolesc Med, 152: 537-539, 1998.
- Schulpen TWJ: The burden of nocturnal enuresis. Acta Pædiatr, 86: 923-926, 1997.
- Hägglöf B, Andrén O, Bergström E et al: Self-esteem before and after treatment in children with nocturnal enuresis and urinary incontinence. Scand J Urol Nephrol, 31 (Suppl 183): 79-82, 1997.
- Butler RJ, Brewin CR, Forsythe WI: Maternal atributions and tolerance for nocturnal enuresis. Behaviour Res Ther, 24: 307-312, 1986.
- Houts AC, Berman JS, Abramson H: Effectiveness of psychological and pharmacological treatments for nocturnal enuresis. J Consult Clin Psychol, 62: 737-745, 1994.
- Bengtsson B, Bengtsson M: Childhood Enuretics in Adult Age: A Long-term, Retrospective Follow up of 88 Children. In Nørgaard JP, Djurhuus JC, Hjälmås K (eds.). Proceedings of the Third International Children’s Continence Symposium. Royal Tunbridge Wells, Wells Medical, pp. 61-63, 1996.
- Butler RJ: Annotation – night wetting in children: psychological aspects. J Child Psychol Psychiat, 39: 453-463, 1998.
- Oredsson AF, Jørgensen TM: Changes in nocturnal bladder capacity during treatment with the bell and pad for monosymptomatic nocturnal enuresis. J Urol, 160: 166-169, 1998.
- Petrican P, Sawan MA: Design of a miniaturized ultrasonic bladder monitor and subsequent evaluation on 41 enuretic patients. IEEE Trans Rehabil Eng, 6: 66-74, 1998.
- Terho P: Desmopressin in nocturnal enuresis. J Urol, 145: 818-820, 1991.
- Hjälmås K, Kruse S, Hellström A-L: Long term desmopressin treatment of children with primary monosymptomatic nocturnal enuresis: an open multicentre study. Brit J Urol, 82: 704-709, 1998.
- Rittig S, Schaumburg H, Schmidt F: Long-term home studies of water balance in patients with nocturnal enuresis. Scand J Urol Nephrol, 31: Suppl 183: 25-26, 1997.
- Miller K, Klauber GT: Desmopressin acetate in children with severe primary nocturnal enuresis. Clin Ther, 12: 357-366, 1990.
- Robson WLM, Nørgaard JP, Leung AKC. Hyponatremia in patients with nocturnal enuresis treated with DDAVP. Eur J Pediatr, 155: 959-962, 1996.
- Bradbury MG, Meadow SR: Combined treatment with enuresis alarm and desmopressin for nocturnal enuresis. Acta Pædiatr, 84: 1014-1018, 1995.
- Hjälmås K, Hellström A-L, Mogren K, Läckgren G, Stenberg A: The overactive bladder in children: a potential future indication for tolterodine. BJU Int, 87: 569-574, 2001.
- Geller B, Reising D, Leonard HL: Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry, 38: 513-516, 1999.
- Von Gontard A, Lehmkul G: Drug therapy of enuresis. Z Kinder Jugenpsychiatr, 24: 18-33, 1996.
- Al-Waili NS: Diclofenac sodium in the treatment of primary nocturnal enuresis: double-blind crossover study. Clin Exp Pharmacol Physiol, 13: 139-142, 1986.
- Kruse S, Hellström A-L, Hjälmås K: Daytime bladder dysfunction in therapy-resistant nocturnal enuresis. A pilot study in urotherapy. Scand J Urol Nephrol, 33: 49-52, 1999.
- Monda JM, Husman DA: Primary nocturnal enuresis: a comparison among observation, imipramine, desmopressin acetate and bedwetting alarm systems. J Urol, 154: 745-748, 1995.
__________________________
Received: September 17, 2001
Accepted: October 12, 2001
_______________________
Correspondence address:
Dr. Kelm Hjälmås
Berzeliigatan 26
SE-412 53 Göteborg, Sweden
Fax: + + (46) (31) 778-9468
E-mail: kelm@kelm.se