SURVIVAL
OF PATIENTS WITH PROSTATE CANCER AND NORMAL PSA LEVELS TREATED BY RADICAL
PROSTATECTOMY
(
Download pdf )
MARCOS F. DALL’OGLIO, ALEXANDRE CRIPPA, ALBERTO A. ANTUNES, LUCIANO J. NESRALLAH, KATIA R. LEITE, MIGUEL SROUGI
Department of Urology , Paulista School of Medicine, Federal University of Sao Paulo, UNIFESP, Sao Paulo, SP, Brazil
ABSTRACT
Introduction:
The unpredictability of prostate cancer has become a daily challenge for
the urologist, with different strategies being required to manage these
cases. In this study, we report on the perspectives for curing prostate
cancer in males undergoing radical prostatectomy with Gleason score of
2-6 on prostate biopsy in relation to pre-operative PSA levels.
Materials and Methods: From 1991 –
2000, we selected 440 individuals whose pathological diagnosis revealed
a Gleason score of 2-6 upon prostate biopsy and who subsequently underwent
retro-pubic radical prostatectomy due to localized prostate cancer. The
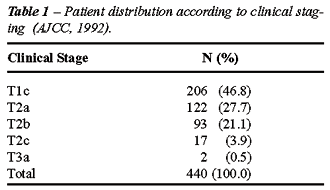
clinical stage identified in the group under study was T1c: 206 (46.8%);
T2a: 122 (27.7%); T2b: 93 (21.1%); T2c: 17 (3.9%); T3a: 2 (0.5%). Following
surgery, we constructed a biochemical recurrence-free survival curve according
to pre-operative PSA levels between 0-4; 4.1-10; 10.1-20 and > 20 ng/mL,
with a median follow-up of 5 years.
Results: Following radical prostatectomy,
the pathological stage was confirmed as pT2a: 137 (31.1%); T2b: 118 (26.8%);
T2c: 85 (19.3%); T3a: 67 (15.2%); T3b: 6 (1.4%); T3c: 22 (5%). The biochemical
recurrence-free survival, according to PSA values between 0-4; 4.1-10;
10.1-20 and > 20 ng/mL, was 86.6%, 62.7%, 39.8% and 24.8% respectively.
Conclusion: Better chances for curing low-grade
prostate cancer occur in individuals with normal PSA for whom a biopsy
is not usually recommended.
Key
words: prostatic neoplasms; prostate-specific antigen; diagnosis;
biopsy; prostatectomy
Int Braz J Urol. 2005; 31: 222-7
INTRODUCTION
Approximately
75% of men over 50 years old are screened for prostate cancer (PCA) through
assessment of the prostate specific antigen (PSA) and digital rectal examination
of the prostate (1). This means there has been an increase in early detection
of PCA with higher likelihood of prostate-confined disease (2), and lower
chances of disease recurrence following treatment, thus reducing mortality
(3). However, there are controversies about the cost-benefit relationship
in PSA screening as well as on the best time for indicating prostate biopsies
in suspected cases. This becomes even more relevant at a PSA range of
from 2.5 to 4 ng/mL. While this is usually considered normal, it can nevertheless
predict detectable cancer. In order to stimulate this debate, recently
Thompson et al. (4) identified PCA in 23.9 % of males with PSA between
2 and 3 ng/mL and 26.9% of males with PSA between 3 and 4 ng/mL.
Due to the heterogeneous features of PCA
and the difficulties for predicting its evolution, this study is intended
to assess individuals with low-risk PCA (defined as Gleason score 2-6
upon biopsy) who underwent radical prostatectomy, and their post-operative
clinical outcomes in relation to the initial PSA levels that led to the
diagnosis.
MATERIALS AND METHODS
This
retrospective study consisted of 440 men clinically diagnosed with PCA,
presenting a Gleason score of 2-6 on prostate biopsy, with a mean age
of 62.5 ± 7.4 years of age (40-79), a mean pre-operative PSA of
8.7 ± 5.6 ng/mL (0.3-32.0), and median follow-up of 60 months (2-130).
Only 4 patients (0.9%) were lost during the follow-up period.
Initial PSA was collected before the prostate
biopsy. During staging, all patients underwent anamnesis and physical
examinations, a dosing of alkaline phosphatase, total and prostatic acid
phosphatase, pelvic computerized tomography and bone scintigraphy in order
to rule out extra-prostatic disease.
All participants underwent radical retro-pubic
prostatectomies with bilateral pelvic iliac lymphadenectomies at our institution
over the period of March 1991 to November 2000. The same surgeon performed
all surgical procedures and pathological analyses were conducted by the
same pathologist.
Clinical staging was defined based on the
American Joint Committee on Cancer classification (5), and histological
grades according to Gleason scores (6).
In selecting the group, we excluded cases
that had received neoadjuvant or adjuvant hormone therapy (14 patients),
and adjuvant radiotherapy (one patient), as well as cases presenting Gleason
scores higher than 6 on biopsy.
Post-operatively, patients were assessed
every 2 months during the first year, then every 6 months for 5 years,
and from then on, yearly. During each assessment, the patient underwent
a digital rectal examination of the prostate cavity and analysis of the
serum PSA. Imaging tests (chest radiography, bone scintigraphy, abdominal
tomography) were repeated every year. Biochemical progression was defined
as a serum PSA equal to or higher than 0.4 ng/mL, a cut-off level that
has been used by other research authors as well (7).
Pre-operative serum PSA was divided into
categories from 0 to 4 ng/mL, 4.1 to 10 ng/mL, 10.1 to 20 ng/mL and higher
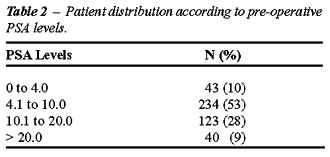
than 20 ng/mL. The patient distribution according to clinical stages is
listed in Table-1, and patients according to pre-operative PSA are listed
in Table-2.
For statistical analysis, we used a survival
analysis approach and considered the biochemical recurrence of disease
an event of interest. This was defined by PSA values equal to or higher
than 0.4 ng/mL. The Kaplan-Meier method and Log-Rank test were used for
the disease-free survival curves. On multivariate analyses, we adjusted
a Cox regression model with proportional risks. A significance level of
5% (p < 0.05) was adopted.
RESULTS
During
a median follow-up of 60 months (2-130.5), 109 (24.8%) of the 440 patients
under study presented biochemical recurrence.
Figure-1 represents a graphic display of
the 440 men under study on different PSA scales, and considering a biochemical
recurrence of the disease as an event of interest. Biochemical recurrence-free
survival rates were 86.6% for PSA values of between 0-4, 62.7% for values
of between 4.1-10, 39.8% for values of between 10.1-20 and 24.8% for values
> 20 ng/mL. We observed that PSA significantly influenced disease-free
survival (p < 0.001). Among the four patients with PSA between 0 and
4.0 who presented recurrence of the disease, two of them had clinical
stage T2a and another 2 had clinical stage T2b. Patient distribution according
to pathological stage is represented in Table-3.
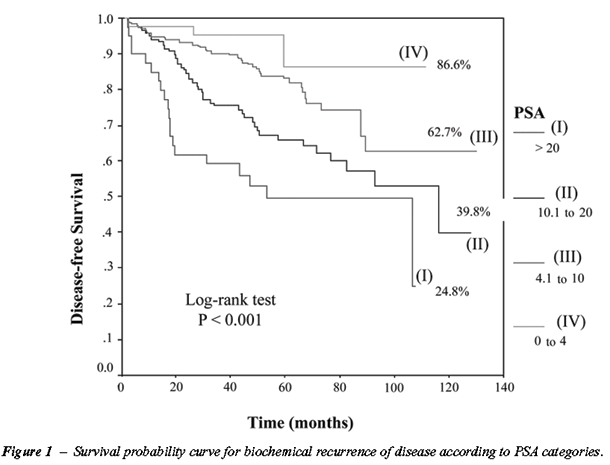
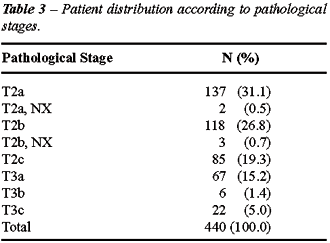
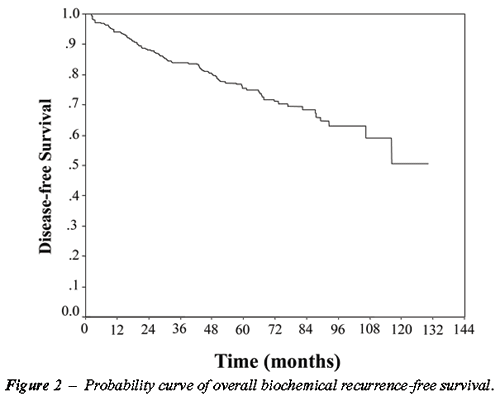
Figure-2 represents the overall biochemical
recurrence-free survival in the group under study.
The analysis of relative risk for PSA in
the Cox regression model reveals that there is no statistical difference
when PSA levels lower than 4.0 ng/mL are compared to PSA levels between
4-10 ng/mL, despite a percentage difference of 23.9%, with serum PSA being
an independent prognostic factor for disease free survival in the post-operative
period (Table-4).

COMMENTS
Our
study showed that the likelihood of biochemical recurrence-free survival
in PCA patients with low Gleason scores, which are usually considered
favorable when these patients undergo radical prostatectomy, should be
looked at more carefully even when the PSA level is lower than 4.
Increasing the dosing of PSA for screening
PCA has enabled early diagnosis and management of this disease, so that
significant changes have occurred in the field over the past 2 decades.
Coincidently with these advancements, technical modifications in radical
prostatectomy techniques have provided lower morbidity, thus reaching
progression-free and cancer-specific survival rates of 68% and 97% respectively
in 10 years (8).
Men with non-palpable PCA detected with
PSA between 2.6 and 4 ng/mL have lower tumoral volume and organ-confined
disease more frequently than those with PSA between 4.1-10 ng/mL (2).
A positive predictive value for PCA between 6.6 and 26.9% was associated
with PSA values between 4-10 ng/mL. Moreover, men diagnosed with localized
PCA with PSA values between 3.1 and 4 ng/mL already present a high-grade
tumor in 25% of cases (9).
The limitations of PSA use and, more specifically,
false positives and false negatives, are well known. Several investigators
have tried to improve the method’s sensitivity and specificity,
including using PSA adjusted by age, as well as PSA density, velocity
and fractions.
The incidence of PCA in individuals with
PSA between 2 and 3 ng/mL is 23.9%, and rises to 26.9% when PSA oscillates
between 3 and 4 ng/mL (4). Such data are in opposition with the findings
of other studies that have identified PCA in men with PSA lower than 4
ng/mL, thus advocating early prostate biopsy.
When stratifying patients that had undergone
radical prostatectomies into PSA categories over 10 years, Roehl et al.
(8) identified biochemical recurrence-free survival rates of 78% –
91% for PSA < 4 ng/mL and 74% for PSA of 4.1-10 ng/mL, while our study
reveals biochemical recurrence-free survival rates over 5 years of 86.6%
and 62.7% for PSA < 4 and 4.1-10 ng/mL respectively.
The probability of identifying PCA when
PSA is between 2.5-4.0 ng/mL in different studies ranges from 20 to 26%
(10,11), and from 24 to 26.5% with PSA between 2-4 ng/mL (12,13) on the
first biopsy and 13% on the second biopsy (13). In the individuals whose
PSA levels oscillate between 2.0-4.0ng/mL, the use of complexed PSA can
improve the specificity and diagnostic sensitivity over the total PSA
total (14), as well as reduce the number of unnecessary biopsies (12).
When performing a study similar to ours, Bhatta-Dhar et al. (15) selected
336 patients with low-risk tumors (PSA < 10 ng/mL, Gleason score =
6, clinical stage T1-2), and after a 6-year follow-up, identified 86%
to 88% biochemical recurrence-free survival.
The probability of the PCA being organ-confined
in individuals with PSA between 2.6-4.0ng/mL is 77%, and 67% with a PSA
between 4.1-10 ng/mL (10). Another study identified 92% of confined PCA
when the PSA was between 2-4 ng/mL (12). These data speak for themselves,
and even with PSA lower than 4.0 ng/mL, extra-prostatic disease is identified
in 23% of cases undergoing curative management. Additionally, with PSA
< 4.0 ng/mL, significant PCA is identified on the biopsy in 67.6% of
cases (12).
We disagree with Bastian et al. (16), who
consider that the majority of non-palpable tumors are insignificant. Our
opinion is that non-palpable tumors can develop aggressive behavior; our
sample reveals biochemical recurrence following treatment in 13.4% of
individuals with PSA lower than 4.0 ng/mL and 27.3% when PSA ranges from
4.1-10 ng/mL.
A possible limitation of our study, we believe,
is that perhaps these data cannot be applied to a non-Caucasian population.
Additionally, since it is a retrospective analysis, sub-staging of Gleason
scores on biopsy could eventually have occurred. However, the fact that
is a homogeneous group with mean follow-up of 5 years and minimal losses
during follow-up can be highlighted as positive factors.
Though the indiscriminate use of PSA can
diagnose insignificant tumors with low biological aggressiveness (17),
it can be a transitory reality. As an example, we point out the study
of PCA patients under careful investigation who, after 3.8 years of follow-up,
showed an elevation in Gleason score in 24% of cases (18). Another study,
conducted by Albertsen et al. (19), which assessed 767 men between 55
and 74 years of age under careful investigation showed that the risk of
death due to progressive disease after 15 years increases according to
the Gleason score in the following proportions: 2-4 (4-7%), 5 (6-11%),
6 (18-30%), 7 (42-70%) and 8-10 (60-87%).
This situation is more dramatic since, despite
the risk of over treating PCA, currently 25% of men undergoing radical
prostatectomy will require a second treatment in the first 5 years following
surgery (20). Based on these data, we wonder if waiting for the PSA to
exceed 4 ng/mL is a correct approach for these men. This could be the
reason many authors discuss the indication of prostate biopsy, assuming
PSA values of 2.6 ng/mL as upper normal level (2.10-13).
CONCLUSIONS
When
considering the variability in the natural course of PCA and the apprehensiveness
of the best moment for indicating prostate biopsy based on PSA, we propose
a change in the paradigm if we wish to increase the real chances of cure,
by indicating prostate biopsy in men with PSA between 2.5-4.0 ng/mL.
_________________________________________
Adriana Sanudo performed the statistical analysis
REFERENCES
- Sirovich BE, Woloshin S, Schwartz LM: Screening men for prostate and colon cancer: are priorities in order? J Gen Intern Med. 2002; 17 (Suppl 1): 212.
- Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y, et al.: Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002; 60:469-7 3; discussion 473-4.
- Chu KC, Tarone RE, Freeman HP: Trends in prostate cancer mortality among black men and white men in the United States. Cancer. 2003; 97: 1507-16.
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al.: Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004; 350:2239-46. Erratum in: N Engl J Med. 2004; 351: 1470.
- Beahrs OH, Henson DE, Hutter RVP: American Joint Committee on Cancer Manual for Staging Cancer. 4a ed. Philadelphia, JB Lippincott. 1992.
- Gleason DF: Histologic Grading and Staging of Prostatic Carcinoma. In: Tannenbaum M, (ed). Urologic Pathology. Philadelphia, Lea & Febiger. 1977; pp. 171-87.
- Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H: The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003; 170: 1872-6.
- Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ: Cancer progression and survival rates following anatomical radical retro-pubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004; 172: 910-4.
- Hernandez J, Thompson IM: Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004; 101: 894-904.
- Antenor JA, Han M, Roehl KA, Nadler RB, Catalona WJ: Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in a longitudinal screening study. J Urol. 2004; 172: 90-3.
- Babaian RJ, Johnston DA, Naccarato W, Ayala A, Bhadkamkar VA, Fritsche HA HA Jr: The incidence of prostate cancer in a screening population with a serum prostate specific antigen between 2.5 and 4.0 ng/mL: relation to biopsy strategy. J Urol. 2001; 165: 757-60.
- Horninger W, Cheli CD, Babaian RJ, Fritsche HA, Lepor H, Taneja SS, et al.: Complexed prostate-specific antigen for early detection of prostate cancer in men with serum prostate-specific antigen levels of 2 to 4 nanograms per milliliter. Urology. 2002; 60 (4 Suppl 1): 31-5.
- Djavan B, Fong YK, Ravery V, Remzi M, Horninger W, Susani M, et al.: Are repeat biopsies required in men with PSA levels < or = 4 ng/mL? A Multi-institutional Prospective European Study. Eur Urol. 2005; 47: 38-44.
- Parsons JK, Brawer MK, Cheli CD, Partin AW, Djavan R: Complexed prostate specific antigen (PSA) reduces unnecessary prostate biopsies in the 2.6-4.0 ng/mL range of total PSA. BJU Int. 2004; 94: 47-50.
- Bhatta-Dhar N, Reuther AM, Zippe C, Klein EA: No difference in six-year biochemical failure rates with or without pelvic lymph node dissection during radical prostatectomy in low-risk patients with localized prostate cancer. Urology. 2004; 63: 528-31.
- Bastian PJ, Mangold LA, Epstein JI, Partin AW: Characteristics of insignificant clinical T1c prostate tumors. A contemporary analysis. Cancer. 2004; 101: 2001-5.
- Epstein JI, Walsh PC, Carmichael M, Brendler CB: Pathologic and clinical findings to predict tumor extent of non-palpable (stage T1c) prostate cancer. JAMA. 1994; 271: 368-74.
- Carter CA, Donahue T, Sun L, Wu H, McLeod DG, Amling C, et al.: Temporarily deferred therapy (watchful waiting) for men younger than 70 years and with low-risk localized prostate cancer in the prostate-specific antigen era. J Clin Oncol. 2003; 21: 4001-8.
- Albertsen PC, Hanley JA, Gleason DF, Barry MJ: Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998; 280: 975-80.
- Lu-Yao GL, Potosky AL, Albertsen PC, Wasson JH, Barry MJ, Wennberg JE: Follow-up prostate cancer treatments after radical prostatectomy: a population-based study. J Natl Cancer Inst. 1996; 88: 166-73.
____________________
Received: April 14, 2005
Accepted: May 28, 2005
_______________________
Correspondence
address:
Dr. Marcos F. Dall’Oglio
Rua Barata Ribeiro, no. 398, 5o. andar
São Paulo, SP, 01308-000, Brazil
Fax: + 55 11 3159-3618
E-mail: marcosdallogliouro@terra.com.br