APPLICATIONS
OF NEUROMODULATION OF THE LOWER URINARY TRACT IN FEMALE UROLOGY
(
Download pdf )
FIROUZ DANESHGARI
Center for Female Pelvic Medicine and Reconstructive Surgery, The Cleveland Clinic Foundation, Cleveland, Ohio, USA
ABSTRACT
Neuromodulation is becoming part of clinical armamentarium for treatment of a variety of lower urinary tract conditions in female urology. Its increased usage stems from need of patients who have exhausted all other therapeutic options for their complex and poorly understood lower urinary tract disorders. Currently neuromodulation may consist of the use of sacral nerve stimulation (SNS) and injectable therapies. Herein, we will discuss the background and development of SNS, its current indications, methods of patient selection and will review the results of the recent published literature on SNS. In addition, we will discuss some of the newer developments in SNS such as Bion device and the future direction in integration of SNS in female urology.
Key
words: bladder; urination disorders; female; neuromodulation;
sacral plexus; electric stimulation
Int Braz J Urol. 2006; 32: 262-72
INTRODUCTION
The
first attempt at electrical stimulation of the lower urinary tract (LUT)
may date back to 1878, when the Danish surgeon Saxtorph treated patients
with urinary retention by intravesical stimulation (1), in which he inserted
a special catheter with a metal electrode transurethrally.
After experimentations with various methods
of simulating the bladder through the transurethral approach, direct detrusor
stimulation (2), pelvic nerve stimulation (3) pelvic floor stimulation
(4), spinal cord stimulation (5), with pioneering work of Tanagho and
later Schmidt (6-9), it was demonstrated that the stimulation of sacral
root S3 generally induces detrusor and sphincter action (10). Following
2 decades of experimentation with various approaches to sacral root stimulation,
finally in October of 1997, sacral neuromodulation for treatment of refractory
urge incontinence was approved by the Food and Drug Administration in
the United States. Since then and at the time of this writing, more than
20,000 of Interstim (Medtronic Inc., Minnesota, Minneapolis, USA) have
been implanted for 3 approved indications of the sacral nerve stimulation
(SNS) of the lower urinary tract.
Herein, we will review the various aspects
of the electrical stimulation of the bladder and its application in management
of the LUT dysfunctions.
MECHANISMS OF ACTION
Neuromodulation of lower urinary tract function can be explained by relatively simple spinal circuits mediating somato-visceral interactions within the sacral spinal cord. It is proposed that SNS activates or “resets” the somatic afferent inputs that play a pivotal role in the modulation of sensory processing and micturition reflex pathways in the spinal cord (11). Urinary retention and dysfunctional voiding can be resolved by inhibition of the guarding reflexes. Detrusor hyperreflexia and the overactive bladder syndrome can be suppressed by one or more pathways, i.e. direct inhibition of bladder preganglionic neurons, as well as inhibition of interneuronal transmission in the afferent limb of the micturition reflex.
PATIENT SELECTION
The
selection of patient for SNS begins with a careful history, physical examination,
routine tests such as urinalysis and urine culture, and most importantly
use of bladder diaries to objectively record voiding variables.
The important elements of history focuses
on the primary voiding variables such as the frequency and severity of
urge incontinent episodes and the number of pads used per 24-hour period.
For patients with refractory urgency frequency, the number of voids, the
voided volumes and the degree of urgency are assessed, and in patients
who experience inefficient voiding or urinary retention, the amount voided
versus catheterized volumes per 24 hours and the patient’s sense
of completeness of evacuation are gathered. A voiding diary is invaluable
in order to objectively document the patient’s voiding habits and
complaints. Urodynamic examination is commonly used to identify the patients
with detrusor overactivity (DO) with or without urinary leakage or urinary
retention. Some reports suggest the utility of the urodynamic studies
(UDS) in identification of proper candidates to SNS (12).
ANATOMICAL LANDMARK AND SURGICAL TECHNIQUES OF SACRAL NEUROMODULATION
Sacral
S3 foramen is the desired anatomical landmark for placement of lead of
the sacral neuromodulation. The techniques for S3 localization have included
manual or fluoroscopic methods. The manual approach includes the palpation
of the sciatic notch, observation for least curved portion of the sacrum,
and measurement of approximately 11 cm from the caudal tip of coccyx (Figure-1).
The manual method is more difficult for obese patients or those without
palpable landmarks. Chai & Mamo introduced the use of “cross-hair”
fluoroscopic technique for S3 localization in 2001 (13). The intent of
the fluoroscopy was not meant to see the S3 foramen, but rather help the
surgeon to identify a specific region to start percutaneous access of
S3 foramen (Figure-2). More importantly, the use of lateral imaging helped
determine the depth required for implanting S3 lead (Figure-3). Use of
fluoroscopy was familiar to surgeons such as urologists as they use of
fluoroscopy in stone surgery and therefore, the application of fluoroscopy
to sacral neuromodulation surgery was quickly accepted. The widespread
use of fluoroscopic localization of S3 later allowed the introduction
of tined S3 lead (14) and transformed the placement of a lead from an
open procedure (15) to a completely percutaneous one. The widely adopted
percutaneous use of tin lead approach abandoned the need for fixation
of the lead by methods such as bone anchors.



Janknegt et al. (16) first described the
staged implantation approach in which an implanted S3 lead, rather than
the temporary lead, was used for initial testing. The staged technique
bypassed the problems with percutaneous needle examination (PNE) which
included a high risk of lead migration and the fact that the original
response of the patient obtained by the temporary wire may have not been
reproduced by the permanent lead. Several reports later confirmed a higher
response rates and lesser rate of lead migration obtained by the staged
approach.
After placement of the lead, the following
sensory and motor responses related to stimulation of the specific sacral
root may be observed:
S2
- Clamp movement or twisting and pinching of the anal sphincter (pulling
down the coccyx).
- Plantar flexion of
the entire foot, lateral rotation.
S3 - Bellows movement of the pelvic floor.
- Plantar flexion of the great
toe(s).
- Parasthesia in the rectum,
perineum, scrotum or vagina.
S4 - Bellows motion of the pelvic floor.
- No lower extremity activity.
- Sensing pulling in the rectum
only.
The desired response and localization for
electrical stimulation of LUT should include S3 responses.
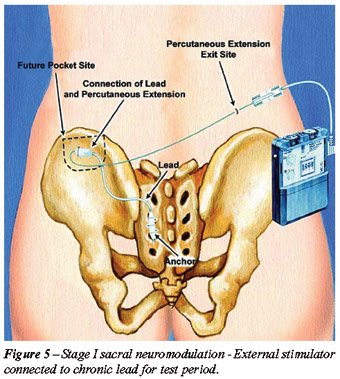
Implantation of SNS consists of 2 steps.
Stage I, or the trial stage, which involves the placement of a stimulation
lead next to the dorsal root of S3 for a test period between 1-4 weeks
(Figure-4). If the patient’s symptoms under the existing list of
indications for SNS improve more than 50% then the patient is a candidate
to undergo the stage II or permanent step in which the permanent implantable
pulse generator (IPG) unit is implanted in the soft tissue of the buttock
of the patient (Figures-5 to 7).



There is no consensus as to whether 1 or
2 implanted S3 leads should be performed as in first stage. Bilateral
implantation allows for testing for both the left and right S3 nerve roots.
A time of the second stage, the side that is less efficacious can be removed
or remain implanted for possible “backup” in case the other
side fails. Currently, there is no evidence that bilateral simultaneous
stimulation has any added benefits to unilateral stimulation. Furthermore,
there is not the ability to stimulate both wires with one IPG in the USA
because the IPG is not a dual channel stimulator. One would need to implant
2 IPGs for bilateral simultaneous stimulation. Nevertheless, bilateral
implantation allows for a more complete evaluation and possibly offers
the patient a higher chance of responding to sacral neurostimulation.
CLINICAL RESULTS
The
reported outcomes of the SNS therefore includes the response of patients
to the stage I (test stage) and to stage II (permanent implantation).
No discussion on the assessment of a treatment
options could be complete without a discussion on the issue of level of
evidence. The evidence required in the medical literature is limited to
data reported in clinical trials, specifically excluding expert opinion.
This is similar to that required to determine the final judgment of a
jury in a legal proceeding, which must be based upon the material evidence
presented during the trial. The judgment (opinion) of the jury is not
evidence. Evidence is factual information presented.
International Consultation on Incontinence
has adopted the Oxford level of evidence as the following categories:
Level 1 - usually involves meta-analysis
of trials, randomized clinical trials or a good quality randomized controlled
trial (RCT) or “all or none” studies in which no treatment
is not an option, for example, vesicovaginal fistula.
Level 2 - includes “low” quality
RCT or meta-analysis of good quality prospective “cohort studies”.
These may include a single group when individuals who develop the condition
are compared with others from within the original cohort group. There
can be parallel cohorts, where those with the condition in the first group
are compared with those in the second group.
Level 3 - evidence includes: A) Good quality
retrospective “case-control studies” where a group of patients
who have a condition are matched appropriately (e.g., for age, sex, etc.)
with control individuals who do not have the condition, B) Good quality
“case series” where a group of patients all, with the same
condition, disease and therapeutic intervention, are described, without
a comparison control group.
Level 4 - evidence includes expert opinion
where the opinion is based not on evidence but on “first principles”
(e.g., physiological or anatomical) bench research. The Delphi process
can be used to give “expert opinion” or greater authority.
In the Delphi process a series of questions are posed to a panel; the
answers are collected into a series of “options”; the options
are serially ranked; if a 75% agreement is reached then a Delphi consensus
statement can be made.
Reports
of Clinical Trials on Urge Incontinence, Urgency / Frequency and
Non-Obstructive Urinary Retention
At this point in time, the SNS has been
approved by the FDA for 3 indications: urge incontinence (UI), urgency
frequency (U/F), and non-obstructive urinary retention (UR). However,
SNS has also been reported to be used for other “off label”
indications, such as neurogenic bladders in multiple sclerosis, interstitial
cystitis, and chronic pelvic pain. Also, there are reports regarding the
possible benefits of bilateral SNS. The majority of the reports on the
non-formally indicated usages of SNS appear in the form of abstracts or
case series.
The initial report on the efficacy of SNS
on treatment of refractory urinary urgent incontinence was reported in
1999 (17) (level 2). This study reported the treatment of 76 patients
with refractory urgent urinary incontinence from 16 contributing worldwide
centers. The patients were randomized to immediate implantation and a
control group with delayed implantation for a six-month period. At six
months, the number of daily incontinence episodes, severity of episodes,
and absorbent pads or diapers replaced daily due to incontinence was significantly
reduced in the stimulation group compared to the delayed group. Of the
34 stimulation group patients, 16 (47%) were completely dry, and an additional
10 (29%) demonstrated a greater than 50% reduction in incontinence episodes.
The interesting finding was that during the therapy evaluation, the group
returned to the baseline level of incontinence when the stimulation was
inactivated. Complications were site pain of the stimulator implantation
in 16%, implants infection in 19%, and leak migration in 7%.
The use of SNS in urgency frequency was
reported in 2000 by Hassouna et al. (18). Similar to the previous design,
51 patients from 12 centers were randomized into an immediate stimulation
group and a control group (25 and 26 patients respectively) (level 2).
Patients were followed for 1, 3 and 6 months, and afterwards at 6-month
intervals up to 2 years. At the 6-month evaluation, the stimulation group
showed improvement in the number of voiding dailies (16.9 ± 9.7
to 9.3 ± 5.1) volume per void (118 ± 74 to 226 ±
124 mL) and degree of urgency (the rank 2.2 ± 0.6 to 1.6 ±
0.9). In addition, significant improvement in quality of life was demonstrated,
as measured by SF-36.
The report of use of SNS in urinary retention
was published in 2001 by Jonas et al. (19), and in this study, 177 patients
with urinary retention refractory to conservative therapy were enrolled
from 13 worldwide centers between 1993 and 1998 (level 2). Thirty-seven
patients were assigned to treatment and 31 to the control group. The follow-up
was done at 1, 3, 6, 12 and 18 months. The treatment group showed 69%
elimination of catheterization at 6 months and an additional 14% with
greater than 50% reduction in catheter volume per catheterization. Temporary
inactivation of SNS therapy resulted in significant increase in residual
volume, but the effectiveness of central nervous stimulation was sustained
for 18 months after implantation.
In 2000, a follow-up report of some of the
above series was published (20) (level 3). This report showed follow-up
results after 3 years in all the approved indications. Fifty-nine percent
of 41 patients had urinary urgent incontinence. Patients showed greater
than 50% with 46% of patients being completely dry. After 2 years, 56%
of the urgency frequency patients showed greater than 50% reduction in
voids per day, and after 1-1/2 years, 70% of 42 retention patients showed
greater than 50% reduction of catheter volume per catheterization.
The results of the use of SNS in the U.S.
patient registry were published in 2002 (21) (level 3). The report included
the use of SNS in 81 patients with all 3 indications: 27 for urgent continence,
10 with urgency frequency and 10 with urinary retention. In this report,
27 from 43 patients with urgent continence, 10 out of 19 with urgency
frequency and 10 out of 19 with urinary retention showed improvement of
more than 50%.
The results of an Italian registry were
published in 2001 (22) (level 3). This report included the reports of
196 patients - 46 males and 150 females - for idiopathic urinary retention.
Fifty percent of patients stopped catheterization and another 13% catheterized
once a day at 1 year after implantation. At the 12-month follow-up, 50%
of patients with hyperreflexia had less than 1 incontinence episode daily
and the problem was completely solved in 66 patients. Of the patients
with urgent continence, 39% were completely dry and 23% had less than
1 incontinence episode daily.
Results of use of SNS in Norway were published
in 2002 (23) (level 3). The author reported the first 3 years of experience
with 53 patients: 45 women and 8 men. This study showed similar results
to previous reported series.
Table-1 shown the published reports of use
of SNS in various conditions of lower urinary tract dysfunction.

Other
Indications
Use of SNS for other off-labeled applications
has been reported for treatment of interstitial cystitis, chronic pelvic
pain, pediatric voiding dysfunction, and neurogenic lower urinary dysfunction
seen in multiple sclerosis. None of the reported case series (level 4)
has led to new approved indications for SNS at the time of this writing.
COMPLICATIONS
A
number of reports have published the complications of the SNS (17-19).
The earlier reports describe the complications with PNE, which is no longer
used in majority of the centers in the United States. Seigel et al. (20)
summarized the complications in patients with refractory urge incontinence,
urgency-frequency and urinary retention that were included in the original
trials of SNS. The complications were divided into both percutaneous test
stimulation related and post implant related problems. Of the 914 test
stimulation procedures done on the 581 patients, 181 adverse events occurred
in 166 of these procedures (18.2% of the 914 procedures). The vast majority
of complications were related to lead migration (108 events, 11.8% of
procedures). Technical problems and pain represented 2.6% and 2.1% of
the adverse events. For the 219 patient who underwent implantation of
the Interstim® system (lead and generator), pain at the neurostimulator
site was the most commonly observed adverse effect at 12 month (15.3%).
Surgical revisions of the implanted neurostimulator or lead system were
performed in 33.3% of cases (73 of 219 patients) to resolve an adverse
event. These included relocation of the neurostimulator because of pain
at the subcutaneous pocket site and revision of the lead for suspected
migration. Explant of the system was performed in 10.5% for lack of efficacy.
Everaert et al. (24) reported the complications
related to SNS itself. Among the 53 patients who had undergone implantation
of the quadripolar electrode (Medtronic Interstim, Model 3886 or 3080)
and subcutaneous pulse generator in the abdominal site (Medtronic Interstim:
Itrel 2, IPG) between 1994 and 1998, device related pain was the most
frequent problem, occurred in 18 of the 53 patients (34%) and occurred
equally in all implantation sites (sacral, flank or abdominal). Pain responded
to physiotherapy in 8 patients and no explantation was done for pain reasons.
Current related complications occurred in 11%. Fifteen revisions were
performed in 12 patients. Revisions for prosthesis related pain (n = 3)
and for late failures (n = 6) were not successful.
Grunewald et al. (25) reported their results
after 4 years of use of SNS (Grunewald 1999). Complications requiring
surgical revisions occurred in 11 of the 37 implanted patients (29.7%).
They included infections in 3 cases (8.1%), lead migration in 2 cases
(5.4%), pain at the site of the implanted pulse generator in 3 cases (8.1%)
and a lead fracture, an electrode insulation defect and skin erosion at
the site of the impulse generator in 1 case (2.7%) respectively.
Hijaz & Vasavada (26) reported the complications
of our group at the Cleveland Clinic Foundation. On hundred eighty stage
I procedures were performed for indications of refractory overactive bladder,
idiopathic and neurogenic urinary retention and interstitial cystitis.
Among this cohort 130 (72.2%) proceeded to stage II implantation of the
implantable pulse generator. In this group, 59 stage I leads were explanted
(27.8%). The majority of lead explants were performed for unsatisfactory
or poor clinical response (46/50; 92%). The rest of the explants were
done for infection (4/50; 8%). Stage one revisions totaled 22 of the 180
stage one (12.2%). Revisions were done for marginal response (13/22),
frayed subcutaneous extension wire (6/22), lead infection (3/22) and improper
localization of stimulus (1/22). Eleven (50%) of the revisions proceeded
for stage two generator implant. When the revision was done for a marginal
response (13/22), the response was ultimately clinically satisfactory
in 5/13 (38.5%) and they proceeded to generator implant. For stage II
complications, explants was performed in 16/130 (12.3%) of the CCF group.
Explants were done for infection and failure to maintain response in 56.3%
and 43.7% respectively. Revisions were done for infection, mechanical
(generator related), and response causes. The revision rate with stage
II was 20% (26/130).
In summary, stage I complications can lead
to either explants or revision of the tined lead. The reasons for either
cause could be related to response of patient, mechanical failure or infection.
Explants for response reasons should not truly be considered a complication
as much as it is an integral part of the procedure. Stag II complications
are also seen for decay of response, mechanical or infection reasons.
Table-2 summarized the common complications of SNS reported in several
series.

Hijaz & Vasavada (26) have also presented
algorithms for trouble shooting of the SNS problems. When infection at
the generator site is diagnosed, the best management would be explanation
of the whole system. Despite attempts to salvage some of these patients,
follow up revealed that the infection persisted in all and eventual explant
was inevitable. Trouble shooting algorithm include search for causes of
a) pocket (IPG site) discomfort; b) recurrent symptoms; c) stimulation
occurring in the wrong area of pelvic; d) no stimulation; and e) intermittent
stimulation.
THE BION DEVICE
In
search for a smaller, lesser invasive and more selective electrical stimulation
of the bladder, use of the Bion devise (Advanced Bionics Corporation,
Valencia CA, USA) in 2 forms (radiofrequency activated bion or RF-bion;
and rechargeable bion or bion-r) have been reported. The Bion device is
a self-contained, battery-powered, telemetrically programmable, current-controlled
mini-neurostimulator with an integrated electrode. It has a size of 27
x 3.3 mm and weighs only 0.7 g. It can be implanted adjacent to the pudendal
nerve at Alcock’s Canal (Figure-8), Bosch, 2005 (27). The results
of the Bion pilot studies indicate that a considerable reduction in the
degree of detrusor overactivity incontinence can be obtained in severely
refractory cases, including women who had failed sacral nerve neuromodulation.
The described technique is well tolerated by the patients. It is minimally
invasive and relatively simple. Clinical trials of the Bion-r device involving
larger numbers of patients are currently under way in the US and Europe.

FUTURE DIRECTIONS
The
initial success of via SNS in treatment of some of the most bothersome
conditions of the bladder has entered the electrical stimulation of the
LUT into the therapeutic armamentarium of physicians dealing with those
conditions. Subsequently, entry of this therapy has introduced new lines
of research to enable us to answer many open and unresolved questions
related to various issues of SNS in clinical practice. Daneshgari and
Abrams compiled a list of the pertinent research questions that in the
opinion of several experts in the area of SNS need to be addressed. Among
those research questions were:
1.
Clinical predictors of responders- it is highly desirable to predict,
with a reasonable level of accuracy, the potential response of the patients
to SNS, thus avoiding the test trial.
2. A comparison between effects of continuous versus intermittent stimulation
with aim of improving the percent of patients benefiting from SNS.
3. Whether a unilateral versus bilateral stimulation in either categories
of the current indications would lead to an improved and more durable
response.
4. Comparing the effects of direct pudendal nerve stimulation versus SNS
in patients with refractory OAB
5. Functional brain imaging of responders and failures after implant of
SNS, to study possible differences in CNS effects of SNS in these 2 groups
6. Animal models to better delineate mechanisms of action for neuromodulation
(i.e. neurotransmitters).
7. Longitudinal study to better understand the interaction between GU,
GI and gynecologic complaints.
As in other areas in medicine, we are looking for those sparks of success that will lead to creative fires of expanding knowledge. But no shortcuts are acceptable. Further use of neuromodulation of the lower urinary tract will have to be examined though the time-tested tools such as properly designed clinical trials as we protect and explore the increasing territory of electrical stimulation of the lower urinary tract.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Madersbacher H: Conservative therapy of neurogenic disorders of micturition. Urologe A. 1999; 38: 24-9.
- Boyce WH, Lathem JE, Hunt LD: Research related to the development of An artificial electrical stimulator for the paralyzed human bladder: A review. J Urol. 1964; 91: 41-51.
- Dees JE: Contraction of the urinary bladder produced by electric stimulation. preliminary report. Invest Urol. 1965; 15: 539-47.
- Caldwell KP: The electrical control of sphincter incompetence. Lancet. 1963; 2: 174-5.
- Nashold BS Jr, Friedman H, Boyarsky S: Electrical activation of micturition by spinal cord stimulation. J Surg Res. 1971; 11: 144-7.
- Nashold BS Jr, Friedman H, Glenn JF, Grimes JH, Barry WF, Avery R: Electromicturition in paraplegia. Implantation of a spinal neuroprosthesis. Arch Surg. 1972; 104: 195-202.
- Schmidt RA, Bruschini H, Tanagho EA: Sacral root stimulation in controlled micturition. Peripheral somatic neurotomy and stimulated voiding. Invest Urol. 1979; 17: 130-4.
- Tanagho EA, Schmidt RA: Bladder pacemaker: scientific basis and clinical future. Urology. 1982; 20: 614-9.
- Tanagho EA, Schmidt RA: Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988; 140: 1331-9.
- Tanagho EA, Schmidt RA, Orvis BR: Neural stimulation for control of voiding dysfunction: a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol. 1989; 142: 340-5.
- Leng WW, Chancellor MB: How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005; 32: 11-8.
- van Kerrebroeck EV, Scheepens WA, de Bie RA, Weil EH: European experience with bilateral sacral neuromodulation in patients with chronic lower urinary tract dysfunction. Urol Clin North Am. 2005; 32: 51-7.
- Chai TC, Mamo GJ: Modified techniques of S3 foramen localization and lead implantation in S3 neuromodulation. Urology. 2001; 58: 786-90.
- Spinelli M, Giardiello G, Gerber M, Arduini A, van den Hombergh U, Malaguti S: New sacral neuromodulation lead for percutaneous implantation using local anesthesia: description and first experience. J Urol. 2003; 170: 1905-7.
- Hohenfellner M, Schultz-Lampel D, Dahms S, Matzel K, Thuroff JW: Bilateral chronic sacral neuromodulation for treatment of lower urinary tract dysfunction. J Urol. 1998; 160: 821-4.
- Janknegt RA, Hassouna MM, Siegel SW, Schmidt RA, Gajewski JB, Rivas DA, et al.: Long-term effectiveness of sacral nerve stimulation for refractory urge incontinence. Eur Urol. 2001; 39: 101-6.
- Schmidt RA, Jonas U, Oleson KA, Janknegt RA, Hassouna MM, Siegel SW, et al.: Sacral nerve stimulation for treatment of refractory urinary urge incontinence. Sacral Nerve Stimulation Study Group. J Urol. 1999; 162: 352-7.
- Hassouna MM, Siegel SW, Nyeholt AA, Elhilali MM, van Kerrebroeck PE, Das AK, et al.: Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol. 2000; 163: 1849-54.
- Jonas U, Fowler CJ, Chancellor MB, Elhilali MM, Fall M, Gajewski JB, et al.: Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol. 2001; 165: 15-9.
- Siegel SW, Catanzaro F, Dijkema HE, Elhilali MM, Fowler CJ, Gajewski JB, et al.: Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology. 2000; 56(6 Suppl 1): 87-91.
- Pettit PD, Thompson JR, Chen AH: Sacral neuromodulation: new applications in the treatment of female pelvic floor dysfunction. Curr Opin Obstet Gynecol. 2002; 14: 521-5.
- Spinelli M, Bertapelle P, Cappellano F, Zanollo A, Carone R, Catanzaro F, et al.: Chronic sacral neuromodulation in patients with lower urinary tract symptoms: results from a national register. J Urol. 2001; 166: 541-5.
- Hedlund H, Schultz A, Talseth T, Tonseth K, van der Hagen A: Sacral neuromodulation in Norway: clinical experience of the first three years. Scand J Urol Nephrol Suppl. 2002; 210: 87-95.
- Everaert K, De Ridder D, Baert L, Oosterlinck W, Wyndaele JJ: Patient satisfaction and complications following sacral nerve stimulation for urinary retention, urge incontinence and perineal pain: a multicenter evaluation. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(4):231-5; discussion 236.
- Grunewald V, Hofner K, Thon WF, Kuczyk MA, Jonas U: Sacral electrical neuromodulation as an alternative treatment option for lower urinary tract dysfunction. Restor Neurol Neurosci. 1999; 14: 189-193.
- Hijaz A, Vasavada S: Complications and troubleshooting of sacral neuromodulation therapy. Urol Clin North Am. 2005; 32: 65-9.
- Bosch JL: The bion device: a minimally invasive implantable ministimulator for pudendal nerve neuromodulation in patients with detrusor overactivity incontinence. Urol Clin North Am. 2005; 32: 109-12.
- Amundsen CL, Webster GD: Sacral neuromodulation in an older, urge-incontinent population. Am J Obstet Gynecol. 2002; 187: 1462-5; discussion 1465.
- Hedlund H, Schultz A, Talseth T, Tonseth K, van der Hagen A: Sacral neuromodulation in Norway: clinical experience of the first three years. Scand J Urol Nephrol Suppl. 2002; (210):87-95.
- Bosch JL, Groen J: Sacral nerve neuromodulation in the treatment of patients with refractory motor urge incontinence: long-term results of a prospective longitudinal study. J Urol. 2000 Apr;163(4):1219-22.
- Shaker HS, Hassouna M: Sacral nerve root neuromodulation: an effective treatment for refractory urge incontinence. J Urol. 1998; 159: 1516-9.
- Aboseif S, Tamaddon K, Chalfin S, Freedman S, Mourad MS, Chang JH, et al.: Sacral neuromodulation in functional urinary retention: an effective way to restore voiding. BJU Int. 2002; 90: 662-5.
_________
Accepted:
October 30, 2005
_______________________
Correspondence
address:
Dr. Firouz Daneshgari
Glickman Urological Institute
The Cleveland Clinic Foundation
9500 Euclid Ave
Cleveland, Ohio, 44195, USA
Fax: + 1 216 444-3680
E-mail: daneshf@ccf.org