DISPOSITION
OF THE STRIATED URETHRAL SPHINCTER AND ITS RELATION TO THE PROSTATE IN
HUMAN FETUSES
(
Download pdf )
LUCIANO A. FAVORITO, LUIS F. P. ALBUQUERQUE, FRANCISCO J. B. SAMPAIO, WALDEMAR S. COSTA
Urogenital Research Unit, State University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil
ABSTRACT
Objective:
To describe the arrangement of the muscle fibers of the striated urethral
sphincter and its relationship with the prostate during the fetal period
in humans.
Materials and Methods: We analyzed 17 prostates
from well preserved fresh human fetuses ranging in age from 10 to 31 weeks
postconception (WPC). Transversal sections were obtained and stained with
Gomori’s trichrome and immunolabeled with anti alpha-actin antibody.
Results: We found that the urethral striated
sphincter (rabdosphincter) is located on the periphery of the smooth muscle
and there was no merge between striated and smooth muscle fibers in any
fetal period. In the prostate apex, the striated sphincter shows a circular
arrangement and covers completely the urethra externally, whereas adjacent
to verumontanum, it looks like a “horseshoe” and covers only
the anterior and lateral surfaces of the urethra. Near the bladder neck,
in fetuses younger than 20 WPC, we have found striated muscle fibers only
at the anterior surface of the prostate, while in fetuses older than 20
WPC, the striated muscle covers the anterior and lateral surfaces of the
prostate.
Conclusions: The urethral sphincter muscle
covers the anterior and lateral surfaces of the urethra in all fetuses
older than 20 WPC, close to the bladder neck and at the distal prostate.
In the region of the prostate apex, the urethral sphincter covers completely
the urethra circularly. The knowledge of the normal anatomy of the urethral
sphincter in fetuses could be important to understand its alterations
in congenital anomalies involving the base of the bladder, the bladder
neck and the proximal urethra.
Key
words: prostate; urethral sphincter; growth and development;
fetuses; anatomy
Int Braz J Urol. 2007; 33: 414-20
INTRODUCTION
Many
anatomic structures are involved in the micturition mechanism such as
the bladder base, the prostatic urethra, the membranous urethra and the
musculature present in those regions. The disposition of the muscular
fibers is important to understand the functional role of those structures
that are part of the so-called sphincteric mechanism, acting both in the
increase of the urethral pressure and during bladder voiding (1-3).
The main areas involved in the process of
urinary continence are located next to the bladder neck and in the distal
portion of the prostatic urethra. The latter is formed by three muscular
layers; two internal layers (longitudinal and circular) of smooth muscle
and another external one (circular) formed by striated muscle, being the
latter the external striated urethral sphincter itself (1,4).
The anatomic relationship between the external
striated urethral sphincter and the prostate apex is decisive in the maintenance
of the urinary continence after radical prostatectomy and in many reconstructive
surgeries, as for example, surgeries for vesical exstrophy (5,6).
The detailed description of the musculature
involved in the urinary continence mechanism is necessary due to the frequency
of surgical manipulations on that region, which can harm the sphincter
(5). The striated sphincter was well described recently through techniques
of computerized reconstruction and magnetic resonance (7-9). The structure
of the striated sphincter, disposed like a collar, and its alterations
during embryo and fetal development could explain the higher incidence
of vesicoureteral reflux in boys when compared to girls (10,11).
The studies that assess the disposition
of the external striated urethral sphincter or the distribution of the
smooth and striated muscle in the initial phases of the human development
are scarce (11,12). The objectives of this work are to describe the localization
and direction of the striated fibers of the external striated urethral
sphincter during the human fetal period and to analyze the relationships
between the smooth and striated muscle layers with the prostatic urethra.
MATERIALS AND METHODS
We
studied 17 blocks containing the prostate, the urethra and the periprostatic
musculature, obtained from well preserved fresh human fetuses, ranging
in age from 10 to 31 weeks postconception (WPC). The fetuses died of causes
unrelated to the urogenital tract and no external evidence of congenital
malformations was detected. The gestational age of the fetuses was estimated
according to the foot length principle (13-16), which is nowadays this
is the most acceptable method to estimate the fetal age. The relationships
between the fetal age, the weight and the vertex-coccyx length (VC) is
shown on Table-1.
The fetal pelvis was carefully dissected
with the aid of a X2.5 magnifying glass. After dissection, the prostate
was removed and immersed in a Bouin solution for 48 to 72 hours. Afterwards
the material was immersed in paraffin and sections of 5 mm thick were
made. The sections were stained with Gomori´s trichrome to verify
the integrity of the specimens and to demonstrate the striated musculature.
The immunostainning procedures were performed
with Zymed® primary monoclonal anti-alpha smooth muscle actin with
appropriate positive and negative controls. Briefly, sections from formalin
fixed, paraffin embedded samples were de-waxed, hydrated in a graded series
of ethanol solutions of decreasing concentrations until the solution was
all water and then washed in phosphate buffered saline (PBS) for 5 minutes.
The sections were treated for 10 minutes with 3% hydrogen peroxide solution
in methanol to block endogenous peroxidase activity. The sections were
washed in three drops PBS, incubated in a humid chamber for 10 minutes
with 1% goat serum, and then incubated a humid chamber with primary antibody
predilute 30 a 60 minuts.
Subsequently, the sections washed in three
drops PBS and incubated at room temperature in a humid chamber with the
biotinylated secondary antibody (Histostain-plus Kits Zymed) for 20 minutes,
washed in three drops PBS and incubated at room temperature in a chamber
with streptavidin- peroxidase-conjugate for 10 minutes. The sections were
washed in three drops PBS and revealed by treating with a 3´3-diaminobenzidine
tetrahydrochloride solution containing 0.1% volume in volume hydrogen
peroxide, and washed in distilled water, dehydrated in an increasing concentration
series of ethanol solutions and mounted with rapid mounting media for
microscopy.
The present study was approved by the Research
Ethics Committee of our institution.
RESULTS
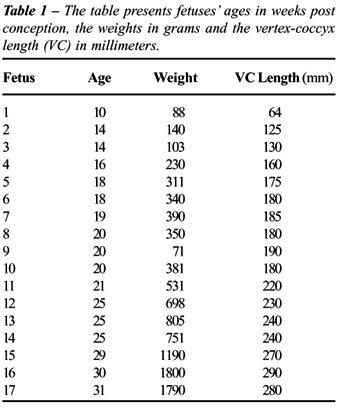
The
striated musculature is the most external layer of the prostate, being
clear the separation of this layer from the extraprostatic adjacent tissue
(Figure-1). Internally, the striated muscle is in contact with the prostate
stroma, mainly formed by collagen and smooth muscle. Also here the transition
is evident even though not in a linear way as it is seen in relation to
the extraprostatic tissue but through extensions both of conective tissue
rich in collagen and the smooth muscle, in direction of the striated muscle.
Various regions present these extensions, however, it was never observed
a mixture between striated and smooth muscle fibers.
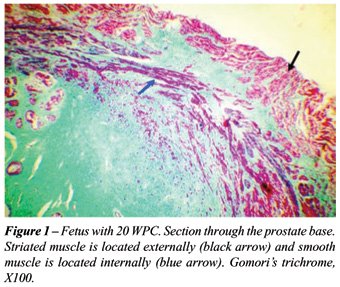
In all fetal ages studied we have detected
muscle fibers extending from the prostate apex to the base. These fibers
did not totally involve the prostate and were more evident anteriorly,
presenting a horseshoe disposition. The muscle fibers were disposed in
an external layer formed by striated muscle and an internal layer formed
by smooth muscle fibers (Figure-2).
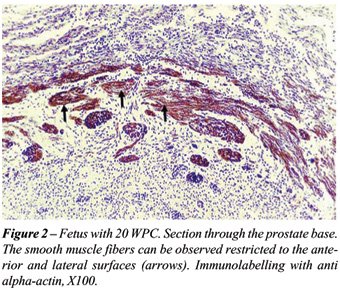
We have found striated fibers in the prostate
lateral surfaces only in fetuses with more than 20 WPC. We did not find
any striated muscle in the mid and proximal posterior surface of the prostate
in none of the cases. At the prostate apex the striated muscle surrounds
all the urethra and its fibers are circularly disposed (Figure-3).
In a fetus with 10 WPC we have observed
an important concentration of muscular fibers restricted to the ventral
region of the prostate (Figure-4). As the fetuses growth we observed an
extension of the muscle fibers for the lateral surfaces of the prostate
with a progressive increase from 18 to 25 WPC (Figure-5). The prostates
of the fetuses with more advanced age presented more smooth muscle in
their lateral surfaces (Figure-6).

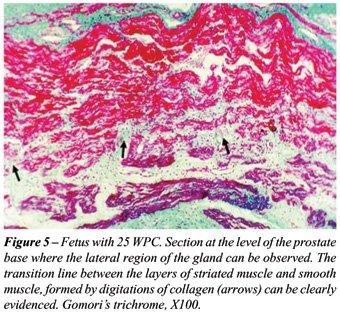
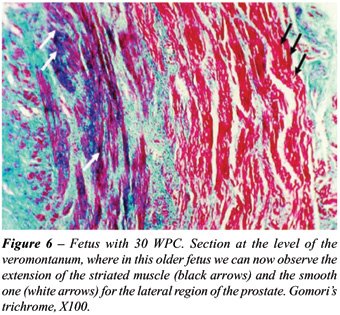
In the specimens where an imunohistochemistry
with anti-alpha actin antibody was performed, that evidence the smooth
musculature, we confirm that the muscular fibers that were not labelled
(striated muscle) were localized more externally. The stained labelled
fibers (smooth muscle), on the other hand were localized more internally
and in close contact with the prostatic capsule (Figure-2).
COMMENTS
The
components of the bladder neck, distal third of the prostatic urethra,
and of the prostate apex actively participate in the process of urinary
continence. The circular smooth muscle of the bladder neck is a direct
extension of the longitudinal layer of the detrusor muscle.
During the bladder distention there is a
strecth of the longitudinal muscular fibers and this increase in tension
is transmitted to bladder neck circular fibers, determining the closure
of the urethra. On the other hand, with the descent of the bladder neck
during micturition, those circular fibers assume an oblique direction
(2). The circular layer of smooth muscle is prominent in the regions of
the prostate base and middle prostate, while the longitudinal layer is
more evident in the distal portion (18). In our study we have observed
a circular smooth muscle layer localized mainly at the prostate base,
while the longitudinal muscle layer was more evident at the prostate apex.
Previous studies have evidenced that the
circular striated fibers of the distal sphincter assume a longitudinal
direction when reaching the lateral surface of the prostate, ascending
to the bladder neck (4,12). Other works show the presence of striated
muscle near the bladder neck, however in the lateral and anterior positions
(2,4,12). In our study we observe near the bladder neck, striated fibers
localized in the prostate anterior and lateral regions. In none of the
sections performed at the base of the protate we observed striated muscle
on its posterior surface.
The striated musculature of the urethral
sphincter surrounds the urethra in the region of the prostate apex, but
does not surround the prostate in the veromontanum level as well as in
the prostate base, presenting a horseshoe aspect, confirming the findings
of previous studies performed in fetuses (11,19). Our findings agree with
those of Yucel & Baskin (11), that evidenced a change in the development
pattern of striated sphincter during the fetal period and the horseshoe
aspect of the musculature in the superior portions of the prostate. Ludwikowski
et al. (10) reported that they did not find changes in the development
pattern of the striated sphincter during the fetal period studied and
did not find at any age the sphincter surrounding the prostatic apex,
that are against our findings.
During the performance of the endoscopic
surgeries of the prostate a resection of the supramontanal portion of
the prostatic urethra is performed, that is the region where generally
occurs the growth of the adenoma (20). The quantity of striated muscle
in this region is inferior to that observed in the inferior portions of
the prostate. The region above the veromontanum is mainly constituted
by smooth muscle, however this musculature is little damaged, since the
smooth muscle is compressed in the direction of the surgical capsule by
the adenoma. In this way, the incidence of incontinence after the transurethral
resection is minimal.
The longitudinal and circular portions of
the striated sphincter form an arch when analyzed together. The urethra
penetrates in the anterior region of the bladder base and descends obliquely
through the prostate crossing this arch of striated muscle. The striated
muscle circular portion is separated from the urethra by mucosal glands
and smooth muscle. The sphincter fibers are transversal in comparison
with the longitudinal fibers of pubovesical ligaments that are placed
anterior to the sphincter, separated from those only by a narrow band
of conjunctive tissue of the retropubic space (5,12,21).
In adults, the anterior and posterior surfaces
of the sphincter are close related to an extensive prostatic vascular
plexus. The integrity of the muscle as a distinctive structure is lost
due to the advancement of the vascular plexus, making it difficult to
describe this structure (12). In fetuses, we found a clear separation
between the striated muscle and the prostate peripheral tissue, similar
to a capsule.
Oerlich (12) describes that the muscle fibers
of the mesenchyme start to present striations in fetuses with 115 mm VC
length, and a complete distinction of striated and smooth fibers is observed
in fetuses with 245mm VC length. We have found striations in all prostates
studied from the age of 10 WPC (64 mm VC length), and those fibers are
different from the smooth muscle fibers in a very evident way. The presence
of sphincteric muscle in the prostatic region in fetuses of the third
trimester was also noticed by Ludwidowski et al. (10) and by Sebe et al.
(19).
Striated muscle fibers are continuous and
inseparable from the smooth fibers of the urethra and interdigitations
occur in the contact plane between the two types of fibers. There is no
kind of fascia between the two areas (12, 22-24). In our sample we have
found the same interdigitations between the two muscular types, even though
no fascial structure has been found.
At the veromontanum level and in the prostate
base we have found an overlay in the lateral surfaces by the striated
urethral sphincter. In the prostate apex, the fibers are disposed with
a circular orientatin, while in the middle prostate did not present a
defined direction. The relationship between the prostate and the external
urethral striated sphincter changes with the prostate development, mainly
concerning their lateral surfaces. With the lateral growth of the lobes,
that depends upon individual characteristics, the striated fibers localized
over the lateral surfaces become more separate. The extension in which
the lateral surfaces will be overlayed by such fibers is variable and
will depend on the development of the lateral lobes(12).
We conclude that the external striated sphincter
surrounds all the urethra at the prostate apex and involves the anterior
and lateral surfaces. Near the bladder neck the musculature is found only
at the anterior face of the prostate in fetuses until 20 WPC, while in
fetuses with more than 20 WPC we can observe an extension of the striated
fibers to the lateral surfaces. The direction of the striated fibers is
predominantly transversal at the prostate apex, at the anterior surface
of the prostate base and at the middle of the prostate. However, at the
prostate lateral region of the middle prostate its disposition is aleatory.
ACKNOWLEDGEMENTS
This research was supported by the National Council of Scientific and Technological Development (CNPq) and by the Rio de Janeiro Foundation for Research Support (FAPERJ), Brazil. Waldemar S. Costa, Luciano A. Favorito and Francisco J. B. Sampaio contributed equally to the research and manuscript preparation.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Presti JC Jr, Schmidt RA, Narayan PA, Carroll PR, Tanagho EA: Pathophysiology of urinary incontinence after radical prostatectomy. J Urol. 1990; 143: 975-8.
- Light JK, Rapoll E, Wheeler TM: The striated urethral sphincter: muscle fibre types and distribution in the prostatic capsule. Br J Urol. 1997; 79: 539-42.
- Whitmore I, Gosling JA, Gilpin SA: A comparison between the physiological and histochemical characterisation of urethral striated muscle in the guinea pig. Pflugers Arch. 1984; 400: 40-3.
- Manley CB Jr: The striated muscle of the prostate. J Urol. 1966; 95: 234-40.
- Myers RP, Goellner JR, Cahill DR: Prostate shape, external striated urethral sphincter and radical prostatectomy: the apical dissection. J Urol. 1987; 138: 543-50.
- Hauri D, Heinzelmann M, Konstantinidis K: Radical prostatectomy in cases of prostatic carcinoma: the problem of postoperative urinary incontinence. Urol Int. 1988; 43: 257-64.
- Myers RP, Cahill DR, Kay PA, Camp JJ, Devine RM, King BF, et al.: Puboperineales: muscular boundaries of the male urogenital hiatus in 3D from magnetic resonance imaging. J Urol. 2000; 164: 1412-5.
- Strasser H, Bartsch G: Anatomy and innervation of the rhabdosphincter of the male urethra. Semin Urol Oncol. 2000; 18: 2-8.
- Brooks JD, Chao WM, Kerr J: Male pelvic anatomy reconstructed from the visible human data set. J Urol. 1998; 159: 868-72.
- Ludwikowski B, Oesch Hayward I, Brenner E, Fritsch H: The development of the external urethral sphincter in humans. BJU Int. 2001; 87: 565-8.
- Yucel S, Baskin LS: An anatomical description of the male and female urethral sphincter complex. J Urol. 2004; 171: 1890-7.
- Oelrich TM: The urethral sphincter muscle in the male. Am J Anat. 1980; 158: 229-46.
- Hern WM: Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet Gynecol. 1984; 63: 26-32.
- Mercer BM, Skalar S, Shariatmadar A, Gillieson MS, D’alton ME: Fetal foot length as a predictor of gestational age. Amer J Obst Gynec, 1987; 156: 350-356.
- Platt LD, Medearis AL, DeVore GR, Horenstein JM, Carlson DE, Brar HS: Fetal foot length: relationship to menstrual age and fetal measurements in the second trimester. Obstet Gynecol. 1988; 71: 526-31.
- Sampaio FJ, Favorito LA: Analysis of testicular migration during the fetal period in humans (10 to 35 weeks postconception). J. Urol. 1988; 159: 540-2.
- Hsu SM, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981; 29: 577-80.
- Sant GR: The anatomy of the external striated urethral sphincter. Paraplegia. 1972; 10: 153-6.
- Sebe B, Schwentner C, Oswald J, Radmayr C, Bartsch G, Fritsch H: Fetal development of striated and smooth muscle sphincters of the male urethra from a common primordium and modifications due to the development of the prostate: an anatomic and histologic study. Prostate. 2005; 62: 388-93.
- Hutch JA, Rambo OS Jr: A study of the anatomy of the prostate, prostatic urethra and the urinary sphincter system. J Urol. 1970; 104: 443-52.
- Haines RW: The striped compressor of the prostatic urethra. Br J Urol. 1969; 41: 481-93.
- Kokoua A, Homsy Y, Lavigne JF, Williot P, Corcos J, Laberge I, et al.: Maturation of the external urinary sphincter: a comparative histotopographic study in humans. J Urol. 1993; 150: 617-22.
- Elbadawi A, Mathews R, Light JK, Wheeler TM: Immunohistochemical and ultrastructural study of rhabdosphincter component of the prostatic capsule. J Urol. 1997; 158: 1819-28.
- Dorschner W, Biesold M, Schmidt F, Stolzenburg JU: The dispute about the external sphincter and the urogenital diaphragm. J Urol. 1999; 162: 1942-5.
____________________
Accepted
after revision:
February 26, 2007
________________________
Correspondence address:
Dr. Luciano A. Favorito
State University of Rio de Janeiro, UERJ
Urogenital Research Unit
Av. 28 de Setembro, No. 87, fundos
Rio de Janeiro, RJ, 20551-030, Brazil
Fax: + 55 21 2587-6121
E-mail: favorito@urogenitalresearch.org