ZOLEDRONIC
ACID EFFECTS INTERLEUKIN-6 EXPRESSION IN HORMONE-INDEPENDENT PROSTATE
CANCER CELL LINES
(
Download pdf )
LAYKA A. ASBAGH, SELIM UZUNOGLU, CAG CAL
Department of Molecular Biology (LAA, SU), Faculty of Science and Arts, Celal Bayar University, Manisa, Turkey, and Department of Urology (CC), School of Medicine, Ege University, Izmir, Turkey
ABSTRACT
Objective:
To investigate the inhibitory effects of zoledronic acid (ZA) on tumor
related growth factor IL-6 in hormone resistant prostate cancer cell lines.
The association between apoptosis and IL-6 inhibition was also assessed.
Materials and Methods: PC-3 and DU145 cell
lines were treated with different concentrations of ZA (1-100µM)
at various intervals (24-72 h.). The cell viability was investigated by
XTT assay and apoptotic effect was evaluated by cell death detection ELISA
kit. Caspase 3/7 activity assay was performed to confirm apoptosis. IL-6
levels were measured by ELISA in the supernatant, and these data were
also confirmed by IL-6 mRNA analysis using RT-PCR.
Results: PC-3 and DU145 cell lines were
sensitive to ZA mediated cytotoxicity in a dose- and time-dependent manner.
However, the apoptotic effect was significantly different among PC-3 and
DU145 cells (p < 0.05). IL-6 secretion was significantly lower in both
cell lines, compared to the untreated control cells (p < 0.05). Although
the increased inhibition of IL-6 secretion was associated with increased
apoptosis in DU145 cells (p = 0.002), there was no similar association
for PC-3 cell line (p = 0.347). When compared to the untreated controls,
the number of cDNA copies was significantly lower in the ZA treated DU145
cell line at doses of 30 and 90µM (p < 0.05), suggesting a reduced
expression of IL-6 mRNA.
Conclusion: ZA exhibited a time- and dose-dependent
apoptotic effect on PC-3 and DU145 prostate cancer cell lines and this
effect was associated with inhibited secretion of IL-6 in DU145 cell line.
Key
words: prostate cancer; zoledronic acid; interleukin-6; experimental
Int Braz J Urol. 2008; 34: 355-64
INTRODUCTION
Prostate
cancer is most common among elderly men, and in 2007, the estimated number
of the newly diagnosed prostate cancer cases was 218.890 in USA (1). Although
local curative treatment strategies are the most appropriate procedures
in organ-confined disease, androgen deprivation therapy represents the
standard treatment in patients with metastatic prostate cancer. Nevertheless,
the development of hormone resistant prostate cancer and progression is
inevitable during androgen deprivation treatment. Unfortunately, no any
other effective and curative alternative treatment has been reported for
these patients.
The new treatment modalities are primarily
focused on growth factors that stimulate the proliferation of prostate
cancer cells. Interleukin-6 (IL-6) is a growth factor for prostate cancer
cells and its high serum levels are known to be directly associated with
clinical prognosis of the disease (2,3). It has also been shown that IL-6
signaling pathway is active and up-regulated in organ-confined prostate
tumors (4) and IL-6 signaling pathway is actively used in metastatic prostate
cancers and hormone independent prostate cancer cell lines.
Several clinical trials have already demonstrated
the beneficial effects of bisphosphonates in prostate cancer patients
(5,6). The growth of metastases may be inhibited by modifying the bone
microenvironment using bisphosphonates. They also exert direct cytotoxic
and apoptotic effects on a variety of human tumor cell lines including
myeloma, breast cancer and prostate cancer (7-9).
Zoledronic acid (ZA) is the most potent
nitrogen containing bisphosphonate compound. It has been shown to inhibit
cell growth and induce apoptosis in prostate cancer cell lines DU145,
PC-3 and LNCaP (10). Current evidence on the effects of ZA suggests that
it is a potential chemotherapeutic agent for the treatment of prostate
cancer, either as monotherapy or in combination. Despite the overwhelming
in vitro studies investigating the anti-tumor activity of the combined
use of ZA with different chemotherapeutics, the molecular targets and
mechanisms of ZA in tumor cells remains a subject of debate.
We hypothesize that ZA may exert its anti-tumor
effect by inhibiting the tumor related growth factor IL-6. Considering
the potential role of IL-6 in the growth regulation of PC-3 and DU145
cell lines, the present study was planned to investigate the relationship
between the anti-tumor activity of ZA and IL-6 secretion in these cells
under in vitro conditions.
MATERIALS AND METHODS
Chemicals
- Cell culture supplies were obtained from Biological Industries (Kibbutz
Beit Haemek, Israel). Zoledronic acid was a generous gift from Novartis
Pharmaceuticals Inc. (Basel, Switzerland). The stock solution of zoledronic
acid was prepared at a concentration of 1 mM in distilled water and aliquots
were stored at -20oC. All other chemicals, unless otherwise mentioned,
were purchased from Sigma Chemical Co (USA).
Cell lines and culture - The androgen-refractory
prostate cancer cell lines, PC-3 and DU145, were preferentially used since
they secrete IL-6 and actively use IL-6 signaling pathway for growth promoting
effects (11) and to maintain resistance to chemotherapy. These cell lines
were kindly provided by Dr. Levent Turkeri from Marmara University, Istanbul,
Turkey. PC-3 and DU145, adherent cell lines were cultured in RPMI 1640,
supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine
and 1% penicillin-streptomycin. All cell cultures were incubated at 37ºC
in 5% CO2
and 95% air.
Cell viability assay - The effects of different
concentrations of zoledronic acid (1,10,30,60,90, and 100µM) on
PC-3 and DU145 cell lines were evaluated by using XTT cell proliferation
kit (Roche Applied Science, Mannheim, Germany). Following the verification
of cell viability by tryptan blue exclusion test, cells were plated on
a 96-well plate in 200µL culture medium at a concentration of 104
cells/well. At 24, 48 and 72 hours of incubation, a 50µL of XTT
labeling mixture was added to each well. The optical density was measured
at 450 nm with a reference wavelength at 650 nm using a microplate reader
(Beckman Coulter, DTX 880 Multimode Reader). The percentage of cytotoxicity
was calculated as follows:

where A is the absorbance.
Evaluation
of apoptosis - The Cell Death Detection ELISA kit (Roche Applied Science,
Mannheim, Germany) was used to detect mono-oligonucleosomes (histone-associated
DNA fragments) as an indicator of apoptosis after zoledronic acid induced
cell death. Briefly, cytoplasmic lysates from untreated controls and zoledronic
acid treated cells were transferred to a streptavidin-coated plate supplied
by the manufacturer. A mixture of Anti-histone-biotine and Anti-DNA-POD
were added to cell lysates and incubated for 2 hours. The complex was
then simultaneously conjugated to form an immune complex on the plate,
which then was read for optical density at 405 nm with a reference wavelength
at 490 nm. The enrichment of mono-oligonucleosomes in cell lysates was
calculated as absorbance of zoledronic acid treated cells/absorbance of
untreated controls.
Caspase 3/7 activity assay - The Caspase-Glo
3/7 assay (Promega, Madison, WI) was used to measure caspase 3/7 activity,
according to the manufacturer’s instructions. PC-3 cells were plated
on a 96-well plate in 100µL culture medium at a concentration of
104 cells/well. After incubation with increasing concentrations
of zoledronic acid, 100µL of Caspase-Glo 3/7 reagent was added to
each well. Then the mixture was incubated for one hour at room temperature
and the luminescence of each sample was measured using a plate-reading
luminometer (Beckman Coulter, DTX 880 Multimode Reader).
Determination of interleukin-6 secretion
- IL-6 levels were quantified in the supernatants of zoledronic acid treated
PC-3 and DU145 cells by using Human IL-6 ELISA Kit (Biosource International
Inc., California, USA). The cells were plated on 24-well plates at a concentration
of 105 cells per well and incubated for 24, 48 and 72 hours
with increasing concentrations of zoledronic acid (1-100µM). Supernatants
were collected for all culture conditions and analyzed for IL-6 levels
using a standard ELISA kit according to manufacturer’s instructions.
Standard curve for quantification was plotted from values of IL-6 standards
provided by kit. IL-6 levels in zoledronic acid treated cells were recalculated
based on the IL-6 levels from untreated control cells at the end of treatment
in order to compensate the differences due to cell number. The decrease
in IL-6 levels was also confirmed by RT-PCR.
Expression of IL-6 mRNA - The effect of
zoledronic acid on IL-6 mRNA level was investigated by RT-PCR. RNA samples
from untreated controls and DU-145 treated cells were isolated by using
High Pure RNA Isolation Kit (Roche Applied Science, Mannheim, Germany).
Primers and probes were included in Roche LightCycler Primer set (Human
Interleukin-6). The procedure was carried out as a single step method
for reverse transcription from RNA to cDNA and subsequent quantification
was made without opening the reaction tube. A Roche Light Cycler apparatus
was used with the following sequence: denaturation at 95ºC for 10
minutes, then 35 cycles of amplifications for 10 s at 95ºC, 10 s
at 68ºC, 16 s at 72ºC, and a final cooling step to 40ºC.
The data were analyzed by the software of Roche LightCycler (1.5) Instrument.
Statistical analyses - All experiments were
set up in triplicate and the results were expressed as mean ± standard
deviation (SD). GraphPad PRISM software (version 5) (San Diego, CA, USA)
was used for the analysis of data and graphic presentations. Student’s
t-test or ANOVA was used for comparisons.
RESULTS
The
cytotoxic and apoptotic effects of zoledronic acid - PC-3 and DU145 cell
lines were sensitive to ZA mediated cytotoxicity; the maximum cytotoxicity
was achieved at 72 hour with 100µM concentration of ZA. The cytotoxicity
was proportional with the increasing concentrations of ZA for both cell
lines and the difference from untreated controls was statistically significant
(p < 0.05). (Data not shown). ZA induced time- and dose-dependent apoptosis
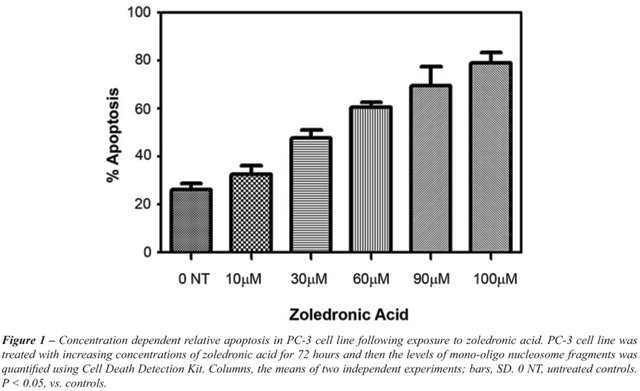
in both cell lines. Data for PC-3 cell line regarding apoptosis is given
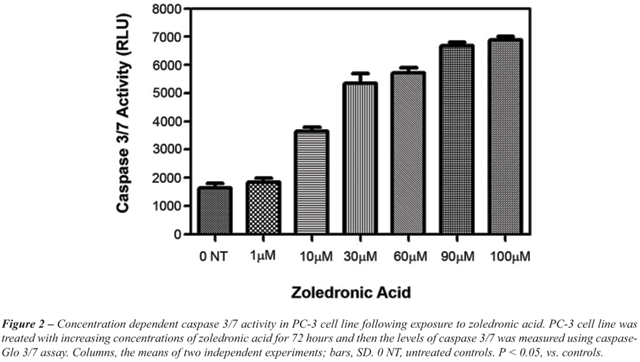
in Figure-1. For PC-3 cell line, Caspase 3/7 activity was significantly
increased in ZA treated cells, compared to untreated controls (p <
0.05) (Figure-2).
IL-6 secretion as detected in the supernatants
of PC-3 and DU145 cells - Incubation of PC-3 and DU145 cells with increasing
concentrations of ZA for 24, 48 and 72 hours resulted in a significant
dose-dependent decrease in IL-6 secretion (p < 0.05) (Figure-3 and
4). This effect was detected with the lowest dose and at the earliest
time points. A difference in terms of dose-dependent inhibition of IL-6
secretion between two cell lines could only be observed at 72 hours. The
lowest level of IL-6 secretion was achieved at 24 hours for PC-3 cells.
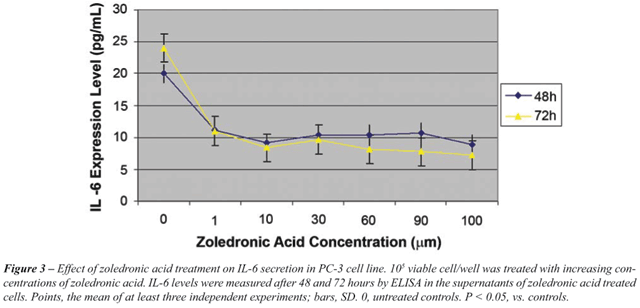
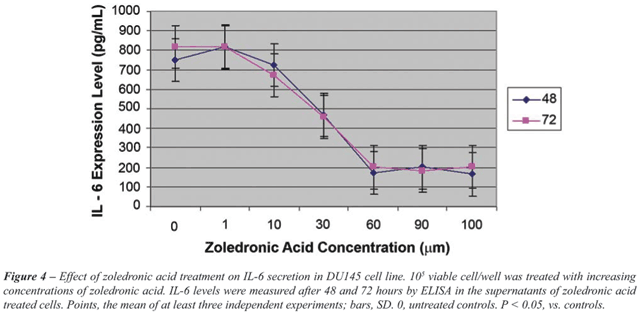
For DU-145 cells, a four-fold decrease in
IL-6 secretion was found in ZA treated cells with 60 µM and higher
concentrations, compared to untreated controls. However, for PC-3 cells,
IL-6 secretion was only halved with the same concentrations and IL-6 secretion
was significantly higher in DU145 cells than PC-3 cells (p < 0.05).
Interestingly, for PC-3 cell line, there
was no association between the degree of apoptosis and inhibition of IL-6
secretion following ZA treatment, (p = 0.347). In contrast , for DU145
cells, the inhibition of IL-6 secretion was correlated with the degree
of apoptosis (p = 0.002).
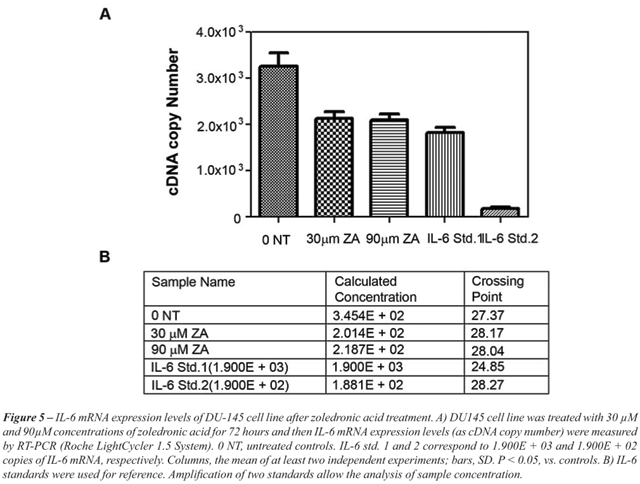
Measurement of IL-6 mRNA levels in ZA treated
DU145 cells by RT-PCR - RT-PCR was performed in DU-145 cells, in order
to examine whether the reduction of IL-6 levels is associated with a decreased
expression of IL-6 mRNA. The number of cDNA copies was significantly lower
in DU-145 cells treated with 30 and 90µM ZA, compared to untreated
controls (p < 0.05) (Figure-5).
COMMENTS
The
present study confirmed that ZA induces apoptosis in PC-3 and DU145 prostate
cancer cell lines in a dose- and time-dependent manner. However, the extent
of this effect was significantly different for PC-3 and DU145 cell lines.
In addition, two cell lines differed in terms of IL-6 secretion. The degree
of apoptosis was not related to the level of the inhibition of IL-6 secretion
for PC-3 cells, which also secrete low levels of IL-6 compared to DU145
cells. On the other hand, the level of reduction in IL-6 secretion was
correlated with the degree of ZA induced apoptosis in DU145 cell lines.
Based on this data, it might be speculated that anti-tumoral effects of
ZA could also be mediated by IL-6 and related signaling pathways in prostate
cancer cells.
It has been well-documented that IL-6 is
a multifunctional cytokine that plays an important role in the regulation
of hematopoiesis, immune response, inflammation, bone metabolism and neural
development (12). It is produced by different cells including lymphoid
or non-lymphoid cells and malignant tissues (13). All prostate cells including
normal prostate epithelia, cells originated from benign prostatic hyperplasia
and malignant prostate cancer are shown to be capable of secreting IL-6
in cell cultures (14). Furthermore, increased secretion of IL-6 ligand
and its receptors in serum has been reported for all stages of prostate
cancer including hormone refractory patients (14,15). Also, clinical prognosis
of prostate cancer is directly affected by serum IL-6 levels (15) and
IL-6 plays an important role for the development of resistance to chemotherapeutics
used in prostate cancer (16). Moreover, exogenous administration of IL-6
has been shown to inhibit doxorubicin-induced apoptosis in PC-3 cells
(17).
In vitro studies demonstrated an increase
in the proliferation of prostate cancer cells with IL-6 stimulation (18,19)
and a decrease in growth rate of androgen insensitive PC-3 and DU145 cell
lines treated with anti-IL-6 antibodies (16). These results suggest that
the combined use of anticancer agents with drugs resulting in an inhibition
of IL-6 expression could increase the efficacy of chemotherapy, particularly
in patients with hormone refractory prostate cancer.
However, conflicting results have been reported
regarding the stimulatory/inhibitory effects of IL-6 on the proliferation
on various prostate cancer cell lines (11,20,21). These differences may
be attributed to a several reasons related to IL-6 signaling pathway.
Firstly, there are membranous and soluble forms of IL-6 ligand and its
receptor, which are strictly regulated. Secondly, gp130, the signal transducer
of IL-6 on the membrane, can be activated by various growth factors. Thirdly,
activated gp130, either simultaneously or preferentially, triggers three
intracellular pathways by the alteration of intracellular domain. IL-6
signaling is mediated by JAK-STAT, ras-raf-MAPK and PI3K-Akt signaling.
It has been suggested that one or two alternative pathways are preferentially
more active in different cell lines. IL-6 can also be up- and down regulated
by autocrine or paracrine effects and feed-back mechanism (11,20,22,23).
Its expression is regulated by several transcription factors such as AP-1,
NFκB, CREB and c/EBP. It is considered that intracellular signaling
pathways of IL-6 also regulate these transcription factors.
Zoledronic acid may affect a some molecules
in signal transduction pathways including cell proliferation process (ras-raf-MAPK),
tumor suppressor genes, apoptotic pathways, cell cycle proteins and posttranslational
processes. Since ZA affects the binding of ras proteins to the membrane
via protein prenylation (10), it might indirectly inhibit cell proliferation.
In a recent study by Cavarretta et al., the effect of IL-6 was shown to
be mediated by oncogene Mcl-1 (myeloid cell leukemia-1), an anti-apoptotic
member of the Bcl-2 family in prostate cell line (24). The association
between ZA treatment and IL-6 secretion may also be regulated by Mcl-1
expression.
Several authors have previously suggested
that ZA cannot induce apoptosis (9,25). Such an inconsistency might be
explained by the differences in ZA concentrations (25) and treatment durations
(9). The present study indicates that a longer treatment period with higher
concentrations of ZA is necessary to induce apoptosis. Interestingly,
when bisphosphonates are combined with other common anti-neoplastic drugs,
a significant synergy occurs. The synergic cytotoxic effect of ZA has
previously been detected on prostate cancer cells (26,27).
Few studies investigated the relation between
IL-6 expression/secretion and ZA treatment. A decreased IL-6 expression
has been reported after ZA treatment in bone marrow stromal cells under
in vitro conditions (28,29). On the contrary, a transient induction of
an increase in TNF-alpha and IL-6 levels with ZA infusion has been demonstrated
in cancer patients with fever (30). Although the disagreements between
the studies may be explained by the variations of in vivo and in vitro
conditions, all of these observations clearly points out that IL-6 has
an important role in the processes related to both bone microenvironment
and metastases in prostate cancer (31). The present study shows a correlation
between the degree of ZA induced apoptosis and the inhibition of IL-6
secretion, implying that the apoptotic effect of ZA is associated with
IL-6 and related pathways. Exogenous administration of IL-6 do not interfere
the anticancer actions of ZA on PC-3 cells, which supports the above-mentioned
association (17).
These findings raise two possible interpretations:
either the reduction of IL-6 secretion itself induces the apoptotic process
or it may be the outcome of ZA induced apoptosis in a dose dependent manner.
If no significant correlation had been found between the decrease in IL-6
expression and the degree of apoptotic process, it could be suggested
that ZA directly inhibits the autocrine mechanisms of IL-6 expression.
It would also be worth mentioning that ZA
may indirectly induce apoptotic mechanisms through affecting signal transduction
pathways on the upstream region of apoptotic pathway. This probability
may explain the reduction of IL-6 secretion with increased apoptosis,
which was observed after ZA treatment in our study. Therefore, it can
be suggested that ZA not only directly induces apoptotic pathways, but
also indirectly affects one or more signal transduction molecules located
on upstream region, which cause the apoptosis in PC-3 and DU145 cell lines.
For these reasons, it is necessary to determine the target molecules that
play key roles on the effects of ZA.
CONCLUSION
The present in vitro study shows a time- and dose-dependent apoptotic effect of ZA on both PC-3 and DU145 prostate cancer cell lines, which correlates with an inhibitory effect on IL-6 expression in DU145 cells. Additional research is required to further elucidate the activity of IL-6 and its role in the pathogenesis of advanced prostate cancer at cellular and molecular levels. Also, further studies are required to investigate the down regulation of oncogene Mcl-1 (myeloid cell leukemia-1), an anti-apoptotic member of the Bcl-2 family, which is regulated directly by IL-6 in ZA treated cells (24). The inhibition of IL-6 with anti-IL-6 antibody sensitizes androgen-independent prostate cancer cells to chemotherapeutic agents in vitro (32); thus, treatment modalities targeting IL-6 may have multiple advantages in prostate cancer patients who receive limited therapeutic and survival benefit from conventional treatment alternatives (18).
CONFLICT OF INTEREST
None declared.
REFERENCES
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 2007; 57: 43-66.
- Culig Z, Steiner H, Bartsch G, Hobisch A: Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005; 95: 497-505.
- Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, et al.: Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000; 6: 2702-6.
- Giri D, Ozen M, Ittmann M: Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001; 159: 2159-65.
- Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO: Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003; 21: 4277-84.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, ET AL.: A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94: 1458-68.
- Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI: Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997; 98: 665-72.
- Fromigue O, Lagneaux L, Body JJ: Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000; 15: 2211-21.
- Lee MV, Fong EM, Singer FR, Guenette RS: Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res. 2001; 61: 2602-8.
- Oades GM, Senaratne SG, Clarke IA, Kirby RS, Colston KW: Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. J Urol. 2003; 170: 246-52.
- Chung TD, Yu JJ, Spiotto MT, Bartkowski M, Simons JW: Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999; 38: 199-207.
- Kishimoto T, Akira S, Taga T: Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992; 258: 593-7.
- Kishimoto T: The biology of interleukin-6. Blood. 1989; 74: 1-10.
- Twillie DA, Eisenberger MA, Carducci MA, Hseih WS, Kim WY, Simons JW: Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995; 45: 542-9.
- Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM: Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001; 58: 1008-15.
- Borsellino N, Belldegrun A, Bonavida B: Endogenous interleukin 6 is a resistance factor for cis-diamminedichloroplatinum and etoposide-mediated cytotoxicity of human prostate carcinoma cell lines. Cancer Res. 1995; 55: 4633-9.
- Tenta R, Tiblalexi D, Sotiriou E, Lembessis P, Manoussakis M, Koutsilieris M: Bone microenvironment-related growth factors modulate differentially the anticancer actions of zoledronic acid and doxorubicin on PC-3 prostate cancer cells. Prostate. 2004; 59: 120-31.
- Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC: Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000; 42: 239-42.
- Borsellino N, Bonavida B, Ciliberto G, Toniatti C, Travali S, D’Alessandro N: Blocking signaling through the Gp130 receptor chain by interleukin-6 and oncostatin M inhibits PC-3 cell growth and sensitizes the tumor cells to etoposide and cisplatin-mediated cytotoxicity. Cancer. 1999; 85: 134-44.
- Okamoto M, Lee C, Oyasu R: Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997; 57: 141-6.
- Mori S, Murakami-Mori K, Bonavida B: Oncostatin M (OM) promotes the growth of DU 145 human prostate cancer cells, but not PC-3 or LNCaP, through the signaling of the OM specific receptor. Anticancer Res. 1999; 19: 1011-5.
- Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, et al.: Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989; 73: 517-26.
- Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, et al.: Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989; 250: 607-10.
- Cavarretta IT, Neuwirt H, Untergasser G, Moser PL, Zaki MH, Steiner H, et al.: The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene. 2007; 26: 2822-32.
- Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al.: Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000; 60: 2949-54.
- Neville-Webbe HL, Rostami-Hodjegan A, Evans CA, Coleman RE, Holen I: Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer. 2005; 113: 364-71.
- Ullen A, Lennartsson L, Harmenberg U, Hjelm-Eriksson M, Kalkner KM, Lennernas B, et al.: Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol. 2005; 44: 644-50.
- Derenne S, Amiot M, Barillé S, Collette M, Robillard N, Berthaud P, et al.: Zoledronate is a potent inhibitor of myeloma cell growth and secretion of IL-6 and MMP-1 by the tumoral environment. J Bone Miner Res. 1999; 14: 2048-56.
- Corso A, Ferretti E, Lunghi M, Zappasodi P, Mangiacavalli S, De Amici M, et al.: Zoledronic acid down-regulates adhesion molecules of bone marrow stromal cells in multiple myeloma: a possible mechanism for its antitumor effect. Cancer. 2005; 104: 118-25.
- Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, et al.: Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res. 2003; 23: 649-54.
- Eaton CL, Coleman RE: Pathophysiology of bone metastases from prostate cancer and the role of bisphosphonates in treatment. Cancer Treat Rev. 2003; 29: 189-98.
- Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET: Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001; 12: 33-40.
____________________
Accepted after revision:
April 4, 2008
_______________________
Correspondence address:
Dr. Cag Cal
Department of Urology
Ege University Tip Fakultesi
Izmir, Turkey
Fax: + 90 232 421-5533
E-mail: cag.cal@ege.edu.tr
EDITORIAL COMMENT
Interleukin-6 (IL-6) is an important regulator of cellular events in human prostate cancer. It has multifunctional effects on proliferation, apoptosis, and angiogenesis and is a target for novel therapies. Most studies were performed with the anti-IL-6 antibody CNTO 328 in vitro and in vivo (1-3). They have demonstrated differences in responsiveness to the antibody between these two different cell lines. The authors of the present paper show that zoledronic acid, that is used for late stage prostate cancer treatment, has a negative effect on IL-6 expression. This is a novel important aspect of action of that drug in human prostate cancer therapy. Since IL-6 is considered a survival factor in some but not all human prostate cancers, this therapy may increase rate of cell death. However, growth-inhibitory effects of IL-6 in selected cell lines were also observed. For that reason, it is important to determine who are the patients who will benefit from anti-IL-6 therapy in the future. In summary, the manuscript by Asbagh et al. is translationally relevant and may stimulate research on IL-6 regulatory effects in prostate cancer in the future.
REFERENCES
- Smith PC, Keller ET: Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate. 2001; 48: 47-53.
- Zaki MH, Nemeth JA, Trikha M: CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004; 111: 592-5.
- Steiner H, Cavarretta IT, Moser PL, Berger AP, Bektic J, Dietrich H, et al.: Regulation of growth of prostate cancer cells selected in the presence of interleukin-6 by the anti-interleukin-6 antibody CNTO 328. Prostate. 2006; 66: 1744-52.
Dr.
Zoran Culig
Department of Urology
Innsbruck Medical University
Innsbruck, Austria
E-mail: zoran.culig@uibk.ac.at