INVERSE
CORRELATION BETWEEN TESTOSTERONE AND VENTRICLE EJECTION FRACTION, HEMODYNAMICS
AND EXERCISE CAPACITY IN HEART FAILURE PATIENTS WITH ERECTILE DYSFUNCTION
(
Download pdf )
EDIMAR A. BOCCHI, VITOR O. CARVALHO, GUILHERME V. GUIMARAES
Laboratory of Heart Failure and Transplantation, Heart Institute, Incor, University of Sao Paulo, SP, Brazil
ABSTRACT
Background:
Neurohormonal activation and abnormalities in growth hormone and testosterone
concentrations have been reported in heart failure (HF). Erectile dysfunction(ED)
is common in these patients and contributes to a low quality of life.
No data are known regarding the correlation between testosterone and hemodynamics,
exercise capacity and cardiac function in HF patients with ED, a marker
of endothelial dysfunction. The aim of this study was to correlate testosterone
levels with cardiac function, hemodynamic and exercise capacity in HF
patients with ED.
Materials and Methods: Fifteen HF patients
underwent a six-minute treadmill cardiopulmonary walking test (6’CWT)
and, ten minutes later, a maximum cardiopulmonary exercise test. Also,
testosterone and other hormones were determined at rest.
Results: Among hemodynamic variables only
diastolic blood pressure on 6’CWT was correlated with testosterone
levels(r =- 0.66, p = 0.007). The variables on exercise tests, VE/VCO2
slope and oxygen consumption did not show any correlation, except the
distance at 6’CWT (r = 0.50, p = 0,047). Right and left ventricle
ejection fraction showed inverse correlation with testosterone (r =- 0.55,
p = 0.03 and r =- 0.69, p = 0.004 respectively).
Conclusion: Testosterone levels correlated
directly with distance at six-minute cardiopulmonary walk test and inversely
with diastolic blood pressure, right and left ventricle ejection fraction
in heart failure patients with erectile dysfunction. Further elucidation
of mechanisms as regards testosterone action in these patients is warranted.
Key
words: heart failure; hemodynamics; physical activity; testosterone;
erectile dysfunction
Int Braz J Urol. 2008; 34: 302-12
INTRODUCTION
Heart
failure (HF) can be considered as the last stage of heart disease and
a significant cause of mortality and morbidity worldwide (1). The left
ventricular systolic dysfunction and limited exercise capacity manifested
by breathlessness and fatigue are determinants of mortality and clinical
events in the follow-up of HF patients. (2,3). Multiple mechanisms have
been reported to be related to exercise capacity including diastolic and
systolic cardiac function, reflex, metabolic, vascular and muscular response
(3). Sexual satisfaction is an important component that influences quality
of life in HF patients. Erectile dysfunction (ED) affects 60 to 70% of
HF clinic outpatients (4). Symptoms, hormonal abnormalities, hemodynamic
status, medication side effects and psychological factors are the major
contributors to this sexual disorder.
In the physiopathology of chronic HF, the
neurohormonal hypothesis for progression of heart failure has been considered
of greater interest than the original hemodynamic mechanisms (5). Activation
of sympathetic nervous system, renin-angiotensin-aldosterone system, arginine
vasopressin, and endothelins are considered as neurohormonal targets in
the treatment of HF. However, other hormonal abnormalities have been reported
in HF such as significant decrease in growth hormones, insulin-like growth
factor I and testosterone concentrations (6). The hormonal and cytokine
activation contributes to peripheral muscle tissue wasting as well as
anabolic/catabolic imbalance (7). In men, testosterone seems to play a
role in determining anabolic function, anti-inflammatory and vasodilator
processes. No data has been reported as regards testosterone’s effect
on hemodynamics, cardiac function and exercise capacity in HF patients
with ED.
The aim of this study was to evaluate the
correlation between serum testosterone levels and cardiac function, hemodynamics,
and exercise capacity in HF patients with ED. In addition, we determined
a HF hormone profile: serum levels of prolactin, luteinizing hormone,
follicle-stimulating hormone, resting norepinephrine, and thyroid hormones.
MATERIALS AND METHODS
Studied
Population
Fifteen randomized chronic male HF patients,
50 ± 10 years with ED (Table-1) for at least four months, in a
steady relationship and presenting interest in sex were included. ED was
defined as the inability to achieve or maintain a durable erection to
permit satisfactory sexual intercourse (the 5th-item of the International
Index of Erectile Dysfunction) (8,9). Patients were in stable clinical
condition for three months without testosterone replacement or drugs that
could have affected testosterone levels, as finasteride, opiates, glucocorticoids
and anticonvulsants. All patients were sedentary and did not have heavy
alcohol consumption, nephrotic syndrome or liver cirrhosis history. All
patients were evaluated for ED etiology such as arterial insufficiency,
venous leakage or penile fibrosis. No etiology was found, except HF syndrome.
The protocol was approved by the Ethical Committee of the Heart Institute.
Subjects provided written informed consent before participation. Exclusion
criteria: ED secondary to causes other than HF, previous ED therapy, recent
use of phosphodiesterase inhibitors, psychiatric or psychological disorders,
unstable angina or recent myocardial infarction, syncope, high-risk arrhythmias,
disease with limitation for exercise except HF, and symptomatic hypotension
or systolic blood pressure (SBP) < 85 mm Hg.

Exercise
Protocol
We measured systolic blood pressure (SBP)
and diastolic blood pressure (DBP) with the patient in an upright position
immediately before each exercise test, at the last minute of the six-minute
walking test (6’WT), at maximum exercise peak, and at 1-minute recovery
(10). Electrocardiography was continuously monitored. Pulmonary ventilation
and gas exchange data were determined on a breath-by-breath basis with
a computerized system (model Vmax 229 Sensormedics). The six-minute walking
test (6’WT) was performed using a programmable treadmill without
inclination and with patient-controlled speed (Series 2000, Marquette
Electronics) at least 2 hours after a light meal and with controlled room
temperature (21°C to 23°C). The patients were oriented to walk
according to Borg’s scale, with exertion level ranging from light
to somewhat hard, from 11 to 13. After return of heart rate (HR), SBP,
DBP, and symptoms to basal condition, patients underwent a progressive
exercise test using a modified Naughton protocol. They were encouraged
to perform maximum exercise until exhaustion or the onset of non tolerated
symptoms occurred and the respiratory exchange ratio exceeded 1.0. The
peak oxygen consumption (peak VO2) was considered the maximum
reached VO2 value.
Cardiac
Function and Hormonal Determinations
The right ventricular and left ventricular
ejection fraction (RVEF and LVEF, respectively), as a percentage, were
determined by echocardiogram. Hormonal dosage at rest before the exercise
test included determination of serum total testosterone (fluoroimmunoassay
method), prolactin, luteinizing hormone, norepinephrine and follicle stimulating
hormone. The normal value of testosterone in this study was 200-950 ng/dL.
Lipid profile assessment was total cholesterol, HDL cholesterol, LDL cholesterol,
Triglycerides. Due to hormonal circadian rhythms, all these tests were
performed in the same period (early morning).
Statistical
Analysis
The descriptive analysis was presented as
mean and standard deviation. The variables studied underwent the non-parametric
Spearman test for correlation, considering p < 0.05 (SPSS Statistical
Software for Windows version 11.5. Inc., Chicago, IL, USA).
RESULTS
All
patients had total testosterone serum levels in the range for normal subjects
(200-950 ng/dL). Four patients had prolactin serum levels below the normal
range (2.5-11.5 ng/mL). One patient had hypothyroidism and the other hyperthyroidism.
The cardiac function, left ventricular diameters, and hormonal values
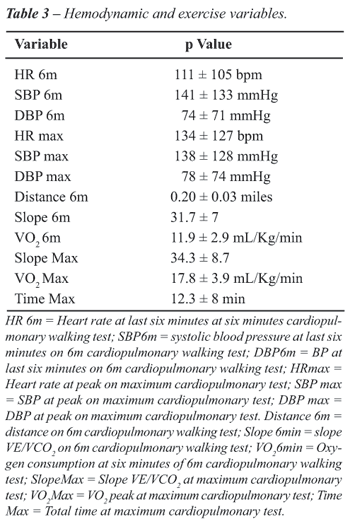
of patients are reported in Table-2. Table-3 shows the hemodynamic and
exercise variables.

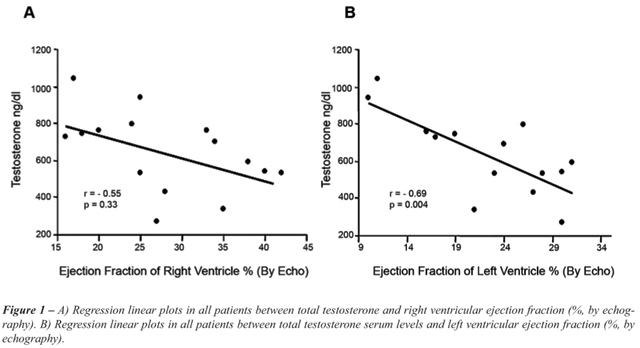
Correlation between Testosterone Serum Levels and Cardiac Diameters,
Cardiac Function, and Hemodynamic Data
Left ventricle ejection fraction (r =- 0.69,
p = 0.004) (Figure-1) and right ventricle ejection fraction (r =- 0.55,
p = 0.03) (Figure-1) showed inverse correlation with testosterone levels.
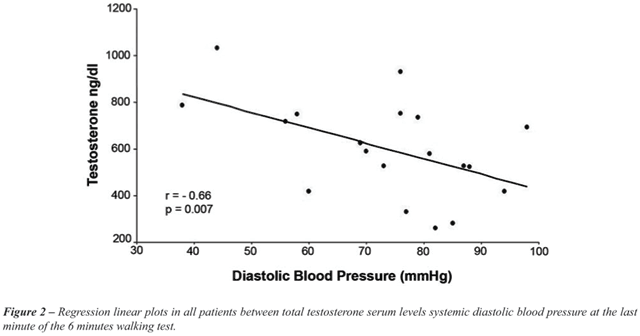
SBP, DBP and heart rate during MCT (maximum cardiopulmonary test) did
not show any correlation with testosterone levels or any hormonal serum
levels except for DBP during the 6´WT that correlated with testosterone
(r =-0.66, p = 0.007) (Figure-2). Left ventricular end diastolic diameter
and left ventricular end systolic diameter did not show any correlation
with testosterone levels either (Table-4).
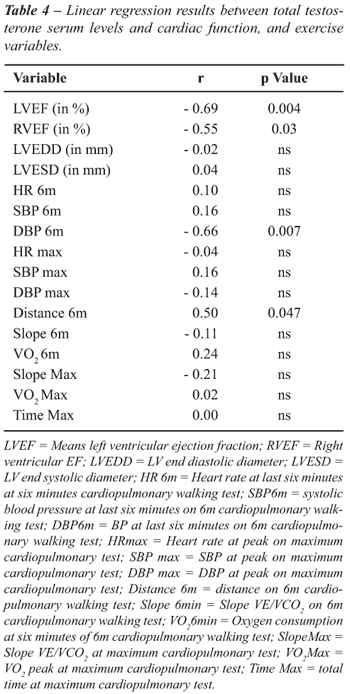
Correlation
between Hormonal Levels and Exercise Capacity Data
Exercise
capacity variables on 6´CWT, VE/VCO2 slope at six minutes,
oxygen consumption at six minutes did not any show correlation with testosterone
levels or hormonal serum levels. Only maximum distance at 6´CWT
showed correlation (r = 0.50, p = 0.047) with testosterone levels. Also
during the MCT, maximum VE/VCO2 slope, maximum oxygen consumption
and time of maximum test did not show correlation either (Table-4)
COMMENTS
Ours
results demonstrated that there was an inverse correlation between right
and left ventricular function and total testosterone serum levels in patients
with ED. Other hemodynamic and exercise variables did not show a correlation
with testosterone serum levels, except diastolic blood pressure and distance
on 6’CWT. Other hormones abnormalities can be found in heart failure
patients with erectile dysfunction.
Low
testosterone levels were reported in severe stroke and acute myocardial
infarction (11). In HF, the testosterone serum levels may be low or in
the normal range depending of the severity of the disease (11). Also,
it has been reported that plasma levels of dehydroepiandrosterone sulfate
decreased in patients with chronic HF in proportion to the severity evaluated
by marker of cardiac function (12). However, to our knowledge this is
the first time that an inverse correlation was demonstrated between left
and right systolic cardiac function and total testosterone serum levels
in selected patients with HF and ED (6,13,14). It is also partially discordant
that, after testosterone administration, serum total levels remained in
the normal physiological range and no significant changes were found in
BNP, TNF-α or LVEF. Also, there was also a positive correlation between
testosterone and cardiac output (15). However, it was concordant with
worsening of left ventricular remodeling with testosterone administration.
The mechanisms to explain our findings are not clear. Effects of testosterone
in the heart are controversial and alternative hypotheses could be proposed.
The
first hypothesis is that the total testosterone serum blood levels could
play a role in pathophysiology of HF, worsening cardiac function. This
is concordant with the concept that anabolic steroids were considered
as having cardiac toxicity with alterations of cellular pathology and
organ physiology similar to those seen with heart failure and cardiomyopathy
(16). In addition, testosterone treatment in very high supra-physiological
doses causes myocardial hypertrophy and stiffening (17). Investigators
administrated physiologic doses of testosterone and found an increase
in the left ventricular diameters, but did not find any change in the
LVEF (18).
The
second hypothesis, in contrast is that testosterone serum levels could
have beneficial effects on HF, and a resistance for testosterone could
be proposed in selected HF patients (6). In rats, androgen therapy has
been reported to improve coronary blood flow and increased both fractional
shortening, thereby improving cardiac function. In addition, animal studies
demonstrated vasodilator effects of androgens with potential beneficial
effects in endothelial dysfunction (19), increment in IGF-1 levels with
reduction in hyperinsulinemia and insulin resistance (20). In addition,
resistance to growth hormone (GH) was also proposed in severe HF patients
based on higher GH concentrations in proportion with low IGF-1 concentration
(21). However, the hypothesis of testosterone resistance needs to be proved
in future investigations including mediators, nongenomic and genomic androgen
action mechanisms (10).
Our
method to include these patients in this study could have influenced our
results because of specific factors related to erectile dysfunction in
HF and higher severity of our patients in comparison with other studies
(18). Patients with HF may experience erectile dysfunction for similar
reasons to the general population, however, there are social, psychological,
physiological, and drug-related consequences specific to HF (22). However,
the influence of ED in our patients should be considered if the concept
that this symptom is a marker of severity of HF and endothelial dysfunction
is accepted (23). Endothelial dysfunction appears to affect all cardiac
and peripheral circulation. Significant relationship between sexual performance
and functional class and six-minute walk test has been reported (24).
Moreover, as suggested for GH resistance in more severe patients, our
results could have been influenced by these factors.
The
correlation between total testosterone and exercise capacity is concordant
with previous randomized, double blind, placebo-controlled parallel trial
of testosterone replacement therapy. Exercise capacity significantly improved
with testosterone therapy, compared with placebo (18). There are evidences
in animal studies that anabolic androgens attenuate muscle fatigue in
response to exercise, although the mechanism has not been identified (25).
In addition, androgens may have different effects on heart and peripheral
muscle (15).
Our
correlation between testosterone and exercise, systemic DBP is in concordance
with vasodilator effect of testosterone, but previous reports have speculated
that for a vasodilatation effect of testosterone supra physiological concentrations
are required (26,27). However, beyond the vessel relaxation testosterone
effect, this may activate the renin-angiotensin system (10,28). Also,
vascular effects could be dose-dependent manner, with opposite effects
according to the dose.
Despite
previous reported improvement in New York Heart Association functional
class and exercise capacity, the potential prescription of testosterone
for HF should be evaluated. This potential prescription should consider
our results and previous study that showed the increment of left diameters
in HF patients after testosterone administration (17). Further investigations
should be performed concerning elucidation of its action in the heart
and to determine if it is safe. Acute hemodynamics, exercise and functional
beneficial effects cannot guarantee a short and long-term benefit in cardiac
function and primary endpoints for heart failure treatment (17,27).
Although
this study is limited by the number of patients and did not include other
markers of HF, nevertheless cardiac function could be considered as one
of the main surrogate endpoints in heart failure. A better elucidation
of testosterone mechanisms and action is warranted in a larger patient
population.
CONCLUSION
Testosterone levels correlated directly with distance at six-minute cardiopulmonary walk test and inversely with diastolic blood pressure, right and left ventricle ejection fraction in heart failure patients with erectile dysfunction.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Bocchi EA, Vilas-Boas F, Perrone S, Caamaño AG, Clausell N, Moreira M da C, Thierer J, et al.: I Latin American Guidelines for the Assessment and Management of Decompensated Heart Failure. Arq Bras Cardiol. 2005; 85 (Suppl 3): 49-94.
- Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al.: Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006; 47: 1997-2004.
- Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, et al.: Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997; 30: 1758-64.
- Jaarsma T, Dracup K, Walden J, Stevenson LW: Sexual function in patients with advanced heart failure. Heart Lung. 1996; 25: 262-70.
- R Ferrara, F Mastrorilli, G Pasanisi, S Censi, N D’aiello, A Fucili, et al.: Neurohormonal modulation in chronic heart failure. Eur Heart J. 2002; 4 (suppl D): D3-D11.
- Kontoleon PE, Anastasiou-Nana MI, Papapetrou PD, Alexopoulos G, Ktenas V, Rapti AC, et al.: Hormonal profile in patients with congestive heart failure. Int J Cardiol. 2003; 87: 179-83.
- Berry C, Clark ALÑ: Catabolism in chronic heart failure. Eur Heart J. 2000; 21: 521-32.
- Wagner G, Saenz de Tejada I: Update on male erectile dysfunction. BMJ. 1998; 316: 678-82.
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM: Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999; 11: 319-26.
- Bocchi EA, Guimarães G, Mocelin A, Bacal F, Bellotti G, Ramires JF: Sildenafil effects on exercise, neurohormonal activation, and erectile dysfunction in congestive heart failure: a double-blind, placebo-controlled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation. 2002; 106: 1097-103.
- Liu PY, Death AK, Handelsman DJ: Androgens and cardiovascular disease. Endocr Rev. 2003; 24: 313-40.
- Moriyama Y, Yasue H, Yoshimura M, Mizuno Y, Nishiyama K, Tsunoda R, et al.: The plasma levels of dehydroepiandrosterone sulfate are decreased in patients with chronic heart failure in proportion to the severity. J Clin Endocrinol Metab. 2000; 85: 1834-40.
- Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M: Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1985; 60: 444-50.
- Jeppesen LL, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K: Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996; 16: 749-54.
- Tappler B, Katz M: Pituitary-gonadal dysfunction in low-output cardiac failure. Clin Endocrinol (Oxf). 1979; 10: 219-26.
- Sullivan ML, Martinez CM, Gennis P, Gallagher EJ: The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998; 41: 1-15.
- Karila TA, Karjalainen JE, Mäntysaari MJ, Viitasalo MT, Seppälä TA: Anabolic androgenic steroids produce dose-dependant increase in left ventricular mass in power atheletes, and this effect is potentiated by concomitant use of growth hormone. Int J Sports Med. 2003; 24: 337-43.
- Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS: Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006; 27: 57-64.
- Pugh PJ, English KM, Jones TH, Channer KS: Testosterone: a natural tonic for the failing heart? QJM. 2000; 93: 689-94.
- Hobbs CJ, Plymate SR, Rosen CJ, Adler RA: Testosterone administration increases insulin-like growth factor-I levels in normal men. J Clin Endocrinol Metab. 1993; 77: 776-9.
- Bocchi E, Moura L, Guimarães G, Conceição Souza GE, Ramires JA: Beneficial effects of high doses of growth hormone in the introduction and optimization of medical treatment in decompensated congestive heart failure. Int J Cardiol. 2006; 110: 313-7.
- Rastogi S, Rodriguez JJ, Kapur V, Schwarz ER: Why do patients with heart failure suffer from erectile dysfunction? A critical review and suggestions on how to approach this problem. Int J Impot Res. 2005; 17 (Suppl 1): S25-36.
- Maguire SM, Nugent AG, McGurk C, Johnston GD, Nicholls DP: Abnormal vascular responses in human chronic cardiac failure are both endothelium dependent and endothelium independent. Heart. 1998; 80: 141-5.
- Jaarsma T, Dracup K, Walden J, Stevenson LW: Sexual function in patients with advanced heart failure. Heart Lung. 1996; 25: 262-70.
- Tamaki T, Uchiyama S, Uchiyama Y, Akatsuka A, Roy RR, Edgerton VR: Anabolic steroids increase exercise tolerance. Am J Physiol Endocrinol Metab. 2001; 280: E973-81.
- Crews JK, Khalil RA: Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999; 19: 1034-40.
- Pugh PJ, Jones TH, Channer KS: Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J. 2003; 24: 909-15.
- Khalil RA: Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005; 46: 249-54.
____________________
Accepted after revision:
April 20, 2008
_______________________
Correspondence address:
Dr. Vitor Oliveira Carvalho
Laboratório de ICC-Transplante
INCOR, HCFMUSP
Av. Dr. Enéas de Carvalho Aguiar
05403-000, Sao Paulo, SP, Brazil
Fax: + 55 11 3069-5419
E-mail: vitor.carvalho@usp.br
EDITORIAL COMMENT
Decreased
testosterone level was reported throughout a 4-year follow-up in elderly
patients with erectile dysfunction (ED) in addition to the association
with an adverse metabolic profile (1). Testosterone deficiency is a common
occurrence in men with chronic heart failure (CHF) and may underpin features
of advanced disease, including reduced skeletal muscle mass and fatigue
(2). Testosterone is known to act as a vasodilator in systemic, coronary
and pulmonary vascular beds, as well as having anabolic properties (3).
This effect could potentially lead to increased cardiac output and improved
cardiovascular function that may contribute to the clinical benefit. Therefore,
administration of testosterone to men with chronic congestive heart failure
may lead to hemodynamic alterations. Furthermore, testosterone deficiency
is positively correlated with cardiac output and exercise capacity in
patients with CHF, whereas a significant improvement in both these parameters
has been observed following testosterone replacement therapy. Testosterone
therapy has also been shown to reduce circulating levels of inflammatory
markers, (TNF-α and IL-1β) in patients with established coronary
artery disease and testosterone deficiency (4).
Although
the mechanisms are poorly understood, the improvement in exercise capacity
was shown to be positively correlated with the increase in serum testosterone
level and was accompanied by a small increase in internal left ventricular
length (2). As testosterone is a vasodilator, this could explain its anti-ischemic
effects on cardiac function during exercise. However, it is currently
unknown whether the vasodilatory effects of testosterone can influence
the fatigability of skeletal muscle in a similar fashion. Therefore, adjunctive
testosterone therapy might augment the positive effects of exercise rehabilitation
on these clinical outcomes in hypogonadal males with stable CHF.
Currently,
the main drawbacks in the design of clinical protocols and the inclusion
of patients for the study of hormonal alteration lie in establishing the
best assay for T measurement and in defining a baseline T cut off level.
Although some limitations of the current study have been mentioned by
the authors, the important feature of this study is that it addresses
one timely and important issue, which is the correlation between serum
testosterone levels and cardiac function, hemodynamic, and exercise capacity
in HF patients with ED.
REFERENCES
- El-Sakka AI, Hassoba HM: Age related testosterone depletion in patients with erectile dysfunction. J Urol. 2006; 176: 2589-93.
- Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS: Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006; 27: 57-64.
- English KM, Jones RD, Jones TH, Morice AH, Channer KS: Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J Endocrinol Invest. 2002; 25: 455-8.
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH: The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004; 89: 3313-8.
Dr.
Ahmed I. El-Sakka
Suez Canal University
School of Medicine
Ismailia, Egypt
E-mail: aielsakka@yahoo.com
Dr.
Farid Saad
Bayer Schering Pharma
Scientific Affairs Men’s Healthcare
Berlin, Germany
EDITORIAL COMMENT
In
this study Bocchi, and his colleagues documented that testosterone (TT)
levels correlated directly with distance at six-minute cardiopulmonary
walk test and inversely with diastolic blood pressure, right and left
ventricle ejection fraction in heart failure patients with erectile dysfunction
(ED). Erectile dysfunction is considered an early sign of endothelial
dysfunction and hence cardiovascular disorders while low TT levels are
related to ED.
TT
level is considered a marker of vascular reactivity and non-traditional
risk factor (Bio Marker) for coronary artery disease and peripheral arterial
disease. It is also related to low arrhythmia threshold and prolonged
QT intervals.
Low
TT level is a predictor of cardiovascular mortality, and marker of low
exercise tolerance and poor quality of life. So if used as an adjuvant
in treatment of heart failure it may significantly improve the functional
capacity and symptoms of heart failure.
Although
the number of cases in this study is limited, as stated by the authors,
the paper is interesting not only to the Urologist and Andrologists but
also to the cardiologist and general physicians. However, 61% of men included
in this study were taking spironolactone, which is an anti-androgen rendering
interpretation of the data a little bit difficult. Understanding the correlation
between TT and cardiac functions will have a great beneficial effect on
ED patients with heart failure.
Further
studies using cardiac speckle technique and tissue Doppler imaging can
provide more accurate assessment of cardiac function and perhaps demonstrate
a correlation between TT levels and cardiac function.
Dr.
Ahmed Asaad
Cardiology Consultant
National Heart Institute
Cairo, Egypt
Dr.
Wael Zohdy
Department of Andrology
Cairo University
Cairo, Egypt
E-mail: wzohdy62@hotmail.com