SUCCESSFUL
TREATMENT OF UNILATERAL CRYPTORCHID BOYS RISKING INFERTILITY WITH LH-RH
ANALOGUE
(
Download pdf )
FARUK HADZISELIMOVIC
Kindertagesklinik, Liestal, Switzerland
ABSTRACT
Introduction:
Infertility is the primary concern for boys with uni- or bilateral undescended
testes. An early and seemingly successful orchiopexy does not improve
fertility in a substantial number of cryptorchid males. We confirmed that
LH-RH analogue (LH-RHa) treatment induces an increase in and maturation
of the germ cells; however, it was uncertain if treatment would improve
the chance of fertility later in life.
Materials and Methods: Thirty unilateral
cryptorchid boys, with an average age of 3 years at the time of surgery,
were included in the study. Testicular biopsy showed that they had impaired
testicular maturation and were therefore at high risk for infertility.
Fifteen of the 30 unilateral cryptorchid boys were treated with 10 µg
LH-RHa (Buserelin) nasal spray, administered on alternate days for a period
of 6 months, following orchiopexy. The control group consisted of 15 cryptorchid
boys who had been treated by Schoemakers type of orchiopexy, alone. After
puberty, the ejaculates of both groups were analyzed.
Results: All males in the untreated group
were severely oligospermic, with 20% being azoospermic. In contrast, 86%
of the treated ex-cryptorchid males had a sperm concentration within the
normal range; this was significantly different from the sperm concentration
found in the untreated group (p = 0.000008).
Conclusion: For the first time, we demonstrate
that infertility in cryptorchidism can be successfully corrected when
suitably treated with a LH-RHa. Sperm parameters normalized following
therapy in the majority of cryptorchid males who, untreated, would have
remained infertile. This innovative hormonal treatment will have a profound
effect on the current recommended surgical treatment of boys with undescended
testes.
Key
words: cryptorchidism; sperm; Ad spermatogonia; fertility; LH-RH
analogue; treatment
Int Braz J Urol. 2008; 34: 319-28
INTRODUCTION
Cryptorchidism
is the most common urogenital birth-defect in males, necessitating surgery
in about 27,000 boys each year in the United States (1). Since 1960, when
Charny stated that “the surgical techniques currently practiced
by most surgeons must improve significantly, albeit the cosmetic results
are arguably better but the functional results fall far short of the intent”
very little has changed (2). Although the effect on testicular development
and fertility has been studied extensively since that time, there is only
one general consensus: untreated boys with bilateral undescended testes
will be infertile.
In 1975, Ludwig and Potempa found that the
fertility rate is inversely proportional to the age of the patient at
the time of surgery. Consequently, it was expected that infertility resulting
from cryptorchidism could be cured if orchiopexy was performed before
the second year of life (3). Thirty years later, when the fertility results
of early surgery were obtainable, we realized that the therapeutic strategy
to operate before the age of 2 years had not improved fertility hopes
in a substantial number of cryptorchid patients (4). Cryptorchid boys
lacking Ad spermatogonia will be infertile despite a seemingly successful
orchiopexy at an early age (4). The main cause is impaired mini-puberty,
the surge of gonadotropins and testosterone that occurs in early infancy
(5). In an attempt to correct impaired mini-puberty, we administered a
LH-RH analogue to cryptorchid boys following successful surgery and achieved
an increase in the number of germ cells (6). We report the results of
the first 15 patients treated with LH-RHa after successful surgery, who
are now young adults, and compare the outcomes with patients who were
treated with surgery only. The amelioration of testicular maturation appears
to result in the normalization of excretory testicular function.
MATERIALS AND METHODS
Fifteen
unilateral cryptorchid boys who had a Schoemakers type of orchiopexy between
the ages of 1-6 years were subsequently treated with LH-RH. Their cryptorchid
testes were located outside of the scrotum since birth. A vast majority
had testes located inguinal or at external inguinal ring. Two patients,
one of each in treated and untreated group had testes located in abdomen.
Twelve out of 15 patients had an unsuccessful HCG treatment before surgery.
Testicular biopsies were obtained during orchiopexy, fixed in 3% glutaraldehyde,
and embedded in Epon. Semi-thin sections, 1 µ thick, were examined
with light microscopy. At least 100 tubular cross sections were counted
to estimate the total number of germ cells per testis. The entire biopsy
was analyzed for the occurrence of Ad spermatogonia. All patients had
< 0.2 germ cells per tubular cross section (normal > 2 per tubular
cross section) and none had Ad (dark) spermatogonia. Therefore, these
patients were at high risk of being infertile (7,8). Within 3 months following
surgery, treatment with the LH-RH analogue Buserelin, a nonapeptide ethilamide
of D-Ser (But)6 LH-RH, was initiated, with 10 µg
applied as an intranasal spray in the evening on alternate days for a
period of 6 months. All patients had regular monthly check-ups during
the course of treatment. The boys’ mothers were questioned about
compliance to the nasal spray treatment as well as possible adverse side
effects of the medication. Furthermore, their general well being and genital
status were estimated, including determining testicular volume, penile
length with a ruler, and Tanner stage of sexual development.
These 15 patients, who are now young adults,
were among the first to receive LH-RH analogue treatment at the ages of
1-6 years (average age, 3 years; 95% CI 2 - 4 years). They were recruited
from a group of 19 who were invited to participate in the study, and were
older than 18 years of age. None of the patients had additional surgeries
or severe illness requiring hospitalization during the 15-19 years following
treatment. Twelve of 15 were non-smokers and none was on chronic medication
or was drug abusers. During a very recent medical check-up, their general
status and Tanner stage of pubertal development was appraised. Testicular
volume and the length of stretched penis were measured. Testicular volume
was determined according to the formula; V=4/3 x π x D/2 x d2;
(0.71 x D x d2). An ejaculate was collected following sexual
abstinence for at least 5 days. Semen analysis was performed by computer-assistance
and additionally confirmed with repeated microscopic examinations. In
accordance with WHO standards, infertility was assumed if the sperm concentration
was < 40 x 106 per ejaculate (9). All patients with a sperm
concentration within the infertile range had at least a second ejaculate
analyzed within 2-4 weeks. The better sperm concentration of the 2 evaluations
was included in the study. The majority (8/15) of patients had to deliver
their sperm specimens by mail; therefore, sperm motility could not be
evaluated. Nevertheless, sperm concentration, ejaculate volumes, and sperm
morphology with teratozoospermic index were evaluated.
The age-matched control group was selected
from 181 unilateral cryptorchid patients who had had their sperm ejaculates
analyzed. Patients in the control group had all undergone a successful
Schoemaker type orchiopexy and a testicular biopsy was obtained during
the surgery; however, they did not undergo additional LH-RH treatment.
Fifteen of the 181 fulfilled the same criteria as the treated group and
were included in the control group. They all had unilateral cryptorchidism,
were the same age at treatment (average age, 4 years; 95% CI 2-6; p =
0.35), had no Ad spermatogonia, and had a total number of germ cells <
0.2 per tubule. 13 out of 15 patients had an unsuccessful HCG treatment
prior to surgery.
Statistical
Analysis
The recruitment of patients for this, non
randomized, study was designed to be balanced. Given the customary values
for the significance level a = 0.05 and the power ß = 90% and using
the module “MTT1-1” in the software “nQuery Advisor”
The sample sizes are determined to N = 12 for a one-sided and to N = 14
for the two-sided Wilcoxon/Mann-Whitney U-test.
Following assumptions were entered into
the module “MTT1-1” of nQuery Advisor: That the mean sperm
count in the treated group was larger than 40 millions whereas in the
untreated group it was smaller than 10 millions where standard deviation
was estimated to be smaller than 20 millions.
We therefore first analyzed the group of
181 ex-unilateral cryptorchid males who were only surgically treated and
identified 15 who fulfilled the entry requirements for the study. Consequently,
we invited our first 19 ex-unilateral cryptorchid males, who had LH-RHa
treatment and were older than 18 years, to participate in the study. Fifteen
of the first consecutive responders entered the study. The Mann-Whitney
U test for unpaired data was used in the software package StatXact 6.30
(2004) from CYTEL Corporation. Nonparametric 95% confidence intervals
for the medians were computed by bootstrapping.
Ethical
Considerations
In accordance with the Helsinki declaration,
the Institutional Review Board (IRB), and the Independent Ethics Committee
of University Children’s Hospital Basel approved all aspects of
this study. In particular, approval was given for research involving the
use of material (data, documents, records or specimens) that had been
collected for non-research purposes.
RESULTS
The
total number of germ cells in the surgery “only” group was
an average of 0.02 germ cells per tubular cross section (95% CI: 0 - 0.2).
This was the same as the number observed in the treated group, who had
an average of 0 germ cells per tubular cross section (95% CI: 0.- 0.05;
p = 0.22). Both groups had no Ad (dark) spermatogonia present in their
entire testicular biopsies. Indicating defective germ cell transformation
due to an impaired mini puberty.
There were no adverse side effects and no
changes in the Tanner stage of pubertal development during the hormonal
treatment.
Most often, mothers reported that their
boys were more active than usual during treatment. Testicular volume remained
unchanged compared with that before the treatment, while there was a significant
increase in penile length from an average of 4.5 cm (95% CI 4-5) before
treatment to an average of 5.0 cm (95% CI 4.5-6) after treatment (p <
0.001) (Table-1).
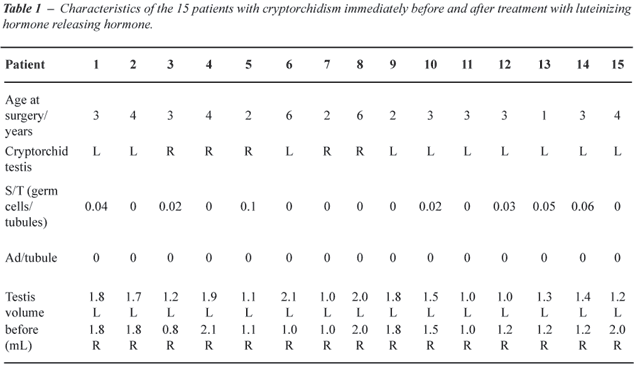
At a recent examination at an average age of
19 years (95% CI 18-22), all males were in a healthy condition. They all
had Tanner V stage of sexual development and normal erectile function.
The length of the stretched penis was in the lower normal limit with average
length of 12 cm (95% CI 10-13 cm) (Table-2). Testicular volume of orchiopexy
testes was less when compared with the contralateral descended partner;
ex-undescended testicle 29 mL (95% CI 22.- 36 mL) vs. contralateral descended
testicle 38 mL (95% CI 30-46 mL; p < 0.0026) (Table-2).

Spermiogram
In the surgery “only” group,
all ex-cryptorchid males suffered severe oligospermia, and 20% (3/15)
of the group had azoospermia. The average sperm concentration was 1 x
106 (95% CI: 0-13) per ejaculate (Figure-1), Table-3.

In the LH-RH treated group, one male had
oligospermia and one had a diminished sperm concentration while in the
remaining 13 males had a normal sperm concentration, (Table-2). Average
total sperm concentration in the treated group was of 90 x 106
(95% CI: 53-164) per ejaculate (Figure-1). This was significantly different
from the sperm concentrations in the surgery “only” group
(p = 0.000008) (Figure-1). Furthermore, in the LH-RHa treated group, the
teratozoospermic index and volume of ejaculates were in the normal range
(Table-2). If fertility capacity were defined according to the Tygerberg
strict criteria, then sperm morphologic pattern was distributed similar
to the distribution found in the normal fertile population (10). Two males
had P-pattern (poor-prognosis), 8 were in the G-pattern (good prognosis)
group, and 5 were in the N-pattern (normal forms > 14%) group (Table-2).

COMMENTS
Attempting
to improve the unfavorable fertility results through early treatment of
cryptorchidism is generally recommended and accepted today. According
to current thinking, hormonal, hormonal/surgical, or only surgical treatment
should be completed before the patient’s second birthday. However,
boys with a severe reduction and impaired transformation of germ cells
regardless of the time of surgery, as well as uni- or bilaterality of
cryptorchid gonads, were infertile (4,11). Therefore, whether or not the
patient will achieve normal fertility following successful surgery depends
mainly upon the presence of Ad spermatogonia at the time of orchiopexy
(4,11). At least half of cryptorchid population undergoing surgery had
no Ad spermatogonia and consequently were candidates for hormonal treatment
(7). It is known that a testis in an intra-abdominal position has in general
bad testicular histology beyond one year of age, and that a testis in
pre-scrotal position has a better or is more close to normal testicular
histology (8,11). However, in all positions, although with different incidences,
the testes with no Ad spermatogonia and < 0.2 germ cell per tubule
could be observed. Thus, the histological findings distinguished those
patients who specifically required additional treatment than testicular
position at surgery, albeit the fact that 90% of boys with intra-abdominal
testes probably require hormonal treatment. In this study only two patients;
one of each group had their testis located in the abdomen, therefore the
study is comparable also with regard to testicular position found at surgery.
In male gonadotropin secretion which increases from 2 to 4 months after
birth stimulating Leydig cells to secrete testosterone (12-14). Testosterone
increase is blunted in cryptorchid boys (13,14). This insufficient testosterone
secretion is responsible for impaired transformation of gonocytes into
Ad spermatogonia (4,5,11,15-17). Additional rationale for LH-RHa treatment
was based on the histological analyses of undescended testes in infancy
(4,5,11,15-20).
The LH-RH analogue (Buserelin) given on
alternate days for a period of five months in a previous study caused
no inhibition of gonadotropin secretion (21). Moreover, LH values determined
in the first morning urine were higher at the end of the treatment (21).
Six months LH-RHa treatment increased the number of germ cells in cryptorchid
testis (7,21). This increase was age dependent (7). The best results were
achieved if the cryptorchid boys were treated before the age of seven
years, implying that successful treatment of impaired mini-puberty should
be performed before this age.
In 1997, we presented results of fertility
outcome in cryptorchid boys who were treated with a LH-RH analogue at
the age of 8 years and older. Compared with surgery as the only treatment,
a significant amelioration, but not normalization, of sperm concentration
was achieved in the treated group (22). This result showed that LH-RHa
treatment had a lasting effect upon spermatogonial development.
To analyze the efficacy of the LH-RHa (Buserelin)
treatment in boys younger than 7 years, we treated unilateral cryptorchid
patients with a high infertility risk according to their testicular histology.
The recruitment of patients for this study was designed to be balanced
[see statistics section]. From 181 ex-unilateral cryptorchid males who
were only surgically treated [representing entire group studied] 15 patients
fulfilled the entry requirements for the study. Consequently, we invited
our first 19 ex-unilateral cryptorchid males, who had LH-RHa treatment
and were older than 18 years, to participate in the study. Fifteen of
the first consecutive responders entered the study Spermiogram results
showed that 13 of 15 unilateral cryptorchid males had a normal sperm concentration.
In addition, the distribution of the fertility patterns of the sperm morphology
was identical to that of the normal fertile population (10). Furthermore,
in those males with a P-pattern morphology, an excellent sperm concentration
will compensate, with a significantly better chance of inducing pregnancy
(23). Normalization of the sperm concentration in 86% of unilateral cryptorchid
males, who were in the high risk group for developing infertility, profoundly
changes our current concept of cryptorchidism treatment. For the first
time, it is possible to demonstrate that infertility caused by cryptorchidism
that was believed to be a congenital malformation can be successfully
corrected if adequately treated.


In conclusion, infertility induced by cryptorchidism
is an endocrine disease of impaired mini-puberty. Treatment with a LH-RHa
before the age of six years following a successful orchiopexy resulted
in the normalization of sperm parameters in the vast majority of patients.
Since not all patients with unilateral cryptorchidism belong to the infertility
risk group, some will profit from early surgery without need for subsequent
LH-RHa treatment. Testicular biopsy is the only diagnostic procedure capable
of identifying patients who need to be treated with LH-RHa following successful
surgery. Because of its important prognostic value, a testicular biopsy
should be routinely performed during the orchiopexy.
ACKNOWLEDGMENT
This study was sponsored in part by the Swiss National Science Foundation. Dr. Mauro Buser, from the Institute for Statistics, Riehen Switzerland, performed the statistical analyses.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Trussell JC, Lee PA: The relationship of cryptorchidism to fertility. Curr Urol Rep. 2004; 5: 142-8.
- Charny CW: The spermatogenic potential of the undescended testis before and after treatment. J Urol. 1960; 83: 697-705.
- Ludwig G, Potempa J: Optimal time for treating cryptorchism (author’s transl). Dtsch Med Wochenschr. 1975; 100: 680-3.
- Hadziselimovic F, Herzog B: The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet. 2001; 358: 1156-7.
- Hadziselimovic F, Zivkovic D, Bica DT, Emmons LR: The importance of mini-puberty for fertility in cryptorchidism. J Urol. 2005; 174: 1536-9; discussion 1538-9.
- Hadziselimovic F, Höcht B: Prospectives. In: Hadziselimovic F (ed.), Cryptorchidism: Management and Implications. Berlin, Springer-Verlag. 1983; pp. 135.
- Hadziselimovic F, Huff D, Duckett J, Herzog B, Elder J, Snyder HM 3rd, et al.: Treatment of cryptorchidism with low doses of buserelin over a 6-months period. Eur J Pediatr. 1987; 146(Suppl 2): S56-8.
- Hadziselimovic F, Hecker E, Herzog B: The value of testicular biopsy in cryptorchidism. Urol Res. 1984; 12: 171-4.
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction, 4th ed. Cambridge, Cambridge University Press. 1999; pp. 60-61.
- Kruger TF, Van der Merwe J, Van Waart J: The Tygerberg Strict Criteria: What Are the Clinical Thresholds for in vitro Fertilization. Intrauterine Insemination, and in vivo Fertilization? In: Kruger TF, Franken DR (eds.). Atlas of Human Sperm Morphology Evaluation. London, Taylor & Francis, 2004:13-18.
- Hadziselimovic F, Hocht B, Herzog B, Buser MW: Infertility in cryptorchidism is linked to the stage of germ cell development at orchidopexy. Horm Res. 2007; 68: 46-52.
- Forest MG, Sizonenko PC, Cathiard AM, Bertrand J: Hypophyso-gonadal function in humans during the first year of life. 1. Evidence for testicular activity in early infancy.J Clin Invest. 1974; 53: 819-28.
- Job JC, Toublanc JE, Chaussain JL, Gendrel D, Roger M, Canlorbe P: The pituitary-gonadal axis in cryptorchid infants and children. Eur J Pediatr. 1987; 146(Suppl 2): S2-5.
- Hamza AF, Elrahim M, Elnagar, Maaty SA, Bassiouny E, Jehannin B: Testicular descent: when to interfere? Eur J Pediatr Surg. 2001; 11: 173-6.
- Hadziselimovic F, Thommen L, Girard J, Herzog B: The significance of postnatal gonadotropin surge for testicular development in normal and cryptorchid testes. J Urol. 1986; 136: 274-6.
- Hadziselimovic F, Emmons LR, Buser MW: A diminished postnatal surge of Ad spermatogonia in cryptorchid infants is additional evidence for hypogonadotropic hypogonadism. Swiss Med Wkly. 2004; 134: 381-4.
- Zivkovic D, Bica DT, Hadziselimovic F: Relationship between adult dark spermatogonia and secretory capacity of Leydig cells in cryptorchidism. BJU Int. 2007; 100: 1147-9; discussion 1149.
- Hadziselimovic F, Herzog B, Huff DS, Menardi G: The morphometric histopathology of undescended testes and testes associated with incarcerated inguinal hernia: a comparative study. J Urol. 1991; 146: 627-9.
- Huff DS, Hadziselimovic F, Snyder HM 3rd, Blyth B, Duckett JW: Early postnatal testicular maldevelopment in cryptorchidism. J Urol. 1991; 146: 624-6.
- Huff DS, Fenig DM, Canning DA, Carr MG, Zderic SA, Snyder HM 3rd: Abnormal germ cell development in cryptorchidism. Horm Res. 2001; 55: 11-7.
- Hadziselimovic F, Hoecht B, Herzog B, Girard J: Does Long Term Treatment with Buserein Improve the Fertility Chances of Cryptorchid Testes? In: Labrie F, Belanger A, Dupont A. (ed.), LH-RH and its Analogues. Amsterdam, Elsevier. 1984.
- Hadziselimovic F, Herzog B: Treatment with a luteinizing hormone-releasing hormone analogue after successful orchiopexy markedly improves the chance of fertility later in life. J Urol. 1997; 158: 1193-5.
- Montanaro Gauci M, Kruger TF, Coetzee K, Smith K, Van Der Merwe JP, Lombard CJ: Stepwise regression analysis to study male and female factors impacting on pregnancy rate in an intrauterine insemination programme. Andrologia. 2001; 33: 135-41.
____________________
Accepted after revision:
May 5, 2008
_______________________
Correspondence address:
Dr. Faruk Hadziselimovic
Kindertagesklinik Liestal
Oristalstrasse 87
Liestal, 4410, Switzerland
Fax:+ 41 61 922-0533
E-mail: praxis.oris@bluewin.ch
EDITORIAL COMMENT
The
present study reinforce the concept that cryptorchidism is resultant of
hormonal alteration that affect both testes, even when the alteration
is unilateral.
The study design evidences that the mere
relocation of an ectopic gonad in the correct anatomic position is not
enough to grant future fertility.
Hormonal therapy for cryptorchidism was
confused with an alternative therapy for surgery. Nevertheless, the main
objective of the hormonal therapy is to improve the histological quality
of the gonad, improving future fertility rates.
This study indicates that hormonal therapy
acts synergically with surgery for obtaining nearly normal fertility rates
in the future.
REFERENCES
- Lee PA, Bellinger MF, Coughlin MT: Correlations among hormone levels, sperm parameters and paternity in formerly unilaterally cryptorchid men. J Urol. 1998; 160: 1155-7; discussion 1178.
- Coughlin MT, Bellinger MF, Lee PA: Age at unilateral orchiopexy: effect on hormone levels and sperm count in adulthood. J Urol. 1999; 162: 986-8; discussion 989.
- Taskinen S, Wikström S: Effect of age at operation, location of testis and preoperative hormonal treatment on testicular growth after cryptorchidism. J Urol. 1997; 158: 471-3.
- Lala R, Matarazzo P, Chiabotto P, Gennari F, Cortese MG, Canavese F, et al.: Early hormonal and surgical treatment of cryptorchidism. J Urol. 1997; 157: 1898-901.
- Bica DT, Hadziselimovic F: Buserelin treatment of cryptorchidism: a randomized, double-blind, placebo-controlled study. J Urol. 1992; 148: 617-21.
Dr.
Marcelo P. Braz
Section of Pediatric Urology
Bonsucesso General Hospital
Rio de Janeiro, RJ, Brazil
E-mail: drmarcelo.braz@gmail.com
EDITORIAL COMMENT
Cryptorchidism is one of the most common congenital pathologies in boys. Treatment of this congenital anomaly concerns the possibility of diminishing risk of malignant degeneration and improving fertility. Surgery is the best treatment for cryptorchidism but there are many studies showing good results for testicular migration after therapy with hCG or with GnRH (1-3). The evidence for the use of hCG vs. GnRH shows advantages for hCG, and a recent review also shows that there is evidence that luteinizing hormone releasing hormone (LH-RH) is more effective than placebo (4) The hormonal treatment in cryptorchidism is controversial. Considering the efficacy and the possible side effects of the hormonal treatment a recent meta-analysis recommended that the hormonal treatment of cryptorchidism could not be further recommended (5). An experimental study shows that hCG impairs the seminiferous tubule histology in normal testes of rats (6). There is still controversy on whether it may be useful as an adjunct to surgery to stimulate germ cells. Current evidence suggests that hormonal therapy may not stimulate transformation of neonatal gonocytes but may trigger prepubertal mitosis of primary spermatocytes (7). Subfertility is considered the main consequence of cryptorchidism even after timely orchiopexy. Gonadotropin-releasing hormone (GnRH) treatment appears to improve fertility later in life by inducing germ cell maturation. A recent paper shows that neoadjuvant GnRH treatment improves fertility index in prepubertal cryptorchidism (8). The great contribution of this paper is the evaluation of seminal parameters in patients that was submitted to orchiopexy with hormonal treatment in childhood. This paper tends to confirm the beneficial effects of medical treatment after orchiopexy in patients with high risk of infertility confirmed by testicular biopsy. One important conclusion of this paper is that testicular biopsy should be performed routinely to evaluate testicular histology during surgery in patients with cryptorchidism.
REFERENCES
- Bica DT, Hadziselimovic F: Buserelin treatment of cryptorchidism: a randomized, double-blind, placebo-controlled study. J Urol. 1992; 148: 617-21.
- Favorito LA, Toledo Filho JS: Study of testicular migration after treatment with human chorionic gonadotropin in patients with cryptorchidism. Braz J Urol, 27: 270-274, 2001
- Gill B, Kogan S: Cryptorchidism. Current concepts. Pediatr Clin North Am. 1997; 44: 1211-27.
- Henna MR, Del Nero RG, Sampaio CZ, Atallah AN, Schettini ST, Castro AA, et al.: Hormonal cryptorchidism therapy: systematic review with metanalysis of randomized clinical trials. Pediatr Surg Int. 2004; 20: 357-9.
- Thorsson AV, Christiansen P, Ritzén M: Efficacy and safety of hormonal treatment of cryptorchidism: current state of the art. Acta Paediatr. 2007; 96: 628-30.
- Kaya C, Karaman MI, Pirincci N, Ozturk M, Yilmazgumrukcu G: Human chorionic gonadotropin deteriorates the histology of rat testes. Urol Int. 2006; 76: 274-7.
- Ong C, Hasthorpe S, Hutson JM: Germ cell development in the descended and cryptorchid testis and the effects of hormonal manipulation. Pediatr Surg Int. 2005; 21: 240-54.
- Schwentner C, Oswald J, Kreczy A, Lunacek A, Bartsch G, Deibl M, et al.: Neoadjuvant gonadotropin-releasing hormone therapy before surgery may improve the fertility index in undescended testes: a prospective randomized trial. J Urol. 2005; 173: 974-7.
Dr.
Luciano A. Favorito
Urogenital Research Unit
State University of Rio de Janeiro
Rio de Janeiro, RJ, Brazil
E-mail: favorito@urogenitalresearch.org