HISTOPATHOLOGICAL
FINDINGS IN EXTENDED PROSTATE BIOPSY WITH PSA ≤ 4 NG/ML
(
Download pdf )
KATIA R. LEITE, MIGUEL SROUGI, MARCOS F. DALL’OGLIO, ADRIANA SANUDO, LUIZ H. CAMARA-LOPES
Laboratory of Medical Investigation - LIM55 (KRL, MS, MFD, AS), Division of Urology, University of Sao Paulo, USP, and Laboratory of Surgical and Molecular Pathology (KRL, LHCL), Sirio Lebanese Hospital, Sao Paulo, SP, Brazil
ABSTRACT
Objective:
Cancer detection has been reported in up to 27% of patients when lowering
the PSA cutoff to 2.5 ng/mL. Although this practice could increase the
number of biopsies performed, it also could lead to more frequent detection
of significant prostate cancers at an organ-confined stage and/or a less
aggressive state. This study describes the incidence of malignancy and
tumor characteristics in extended prostate biopsies with PSA ≤
4 ng/mL.
Materials and Methods: Prostate biopsies
from 1081 patients where examined, 275 (25.4%) patients had PSA level
≤ 4 ng/mL.
Results: Cancer was diagnosed in 32.0% and
35.7% of patients with PSA ≤
4 ng/mL and > 4 ng/mL, respectively (p = 0.906). The median Gleason
score was 7 independent of PSA > or ≤
4 ng/mL (p = 0.078). The median number of cores positive for tumor was
4 and 3, respectively, for PSA > 4 ng/mL and PSA ≤
4 ng/mL (p = 0.627). There was a difference in the total percent of tumors
involving all cores, 11% and 7% for PSA > or ≤
4 ng/mL (p = 0.042). Fifty-six patients underwent radical prostatectomy,
12 had PSA ≤ 4 ng/mL. In both
groups, a diagnosis of cancer was accurate with no differences in Gleason
score, tumor volume or staging for both groups.
Conclusion: When PSA is below 4 ng/mL, cancer
is detected in a proportion equal to the proportion diagnosed with a PSA
> 4 ng/mL, and tumor characteristics are similar between the two groups.
Only clinically significant tumors were diagnosed following radical prostatectomy.
Key
words: PSA; prostate cancer; biopsy; diagnosis; Gleason score; tumor volume
Int Braz J Urol. 2008; 34: 283-92
INTRODUCTION
Numerous
investigators have demonstrated the detection of an increasing proportion
of early-stage prostate cancer (CaP) and improvement in biochemical outcome
after treatment in the Prostate-specific antigen (PSA) era (1-4). It is
also believed to be at least partially responsible for the recent decline
in prostate cancer mortality rates in the US and in some European countries
(5,6).
Traditionally, a PSA cutoff of 4.0 ng/mL
has been used to recommend prostate biopsy (7). However, one third of
men with PSA level between 4 and 10 ng/mL and more than one half with
PSA greater than 10 ng/mL are found to have cancer that has extended to
the surgical margins or to the extraprostatic tissue (8).
When the PSA cutoff level is lowered to
2.5 ng/mL, the cancer detection rate has been reported to be up to 27%.
Although a PSA threshold of less than 4.0 ng/mL may increase the number
of biopsies performed, studies have shown that it also leads to more frequent
detection of significant CaP at an organ-confined stage and/or a less
aggressive state with no excessive increase in the detection of clinically
insignificant cancers (9-12).
Another matter of debate is the contemporary
strategy of extended prostate biopsy, which increases the number of needle
cores from 8 to 13, which is a practice that could lead to a greater detection
of clinically insignificant cancers. Conversely studies have shown that
this practice is responsible for an increase of more than 30% in cancer
detection not related to clinically insignificant cancer (13).
Histopathological findings and tumor characteristics
have not been well characterized when the PSA cut-off is below 4 ng/mL
in the extended prostate biopsy era. To our knowledge complete data including
tumor volume have not been previously reported. The aim of this study
was to compare the histopathological findings of extended prostate biopsy
and radical prostatectomy in men with PSA levels lower or higher than
4 ng/mL.
MATERIALS AND METHODS
From
January 1st 2005 to October 31st 2006, 1587 biopsies
were examined in our laboratory. All information was available for 1081
patients. The mean age was 61.7 years, median 61 (range 31-93). The mean
PSA was 7.43 ng/mL, median 5.5 ng/mL (range 0.3-146.0 ng/mL). The mean
size of the prostate was 57.6 cm3, median 48 cm3
ranging from 15 to 275 cm3, and the mean number of cores taken
in each biopsy section was 15.5, median 14, ranging from 6 to 47.
Of the 1081 patients, 275 (25.4%) had PSA
levels ≤ 4 ng/mL. The median age was 59 years (range 31-78), the
mean size of the prostate was 40.6 cm3 (SD 21.5) and the median
number of cores taken in each biopsy section was 14, ranging from 6 to
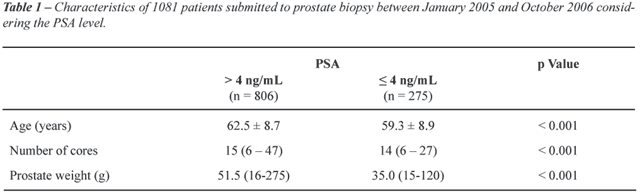
27. The characteristics of the patients according the PSA levels are in
Table-1.
The reason for the biopsy in the men with
PSA under 4 ng/mL was available for only 71 (25.8%) patients. Abnormalities
in the digital rectal examination was the primary cause, described in
33 (46.5%) patients, followed by suspicious or pre-malignant (prostate
intraepithelial neoplasia (PIN) and atypical small acinar proliferation
(ASAP)) lesion in previous biopsies in 27 (38.0%), persistent elevation
of PSA in 5 (7.1%), family history of prostate cancer in 4 (5.6%) and
cancer previously diagnosed in transurethral resection in 2 (2.8%).
Transrectal ultrasound guided prostate biopsies
were routinely processed and examined by only one pathologist (KRL). Diagnosis
was classified as: 1) benign; 2) suspicious but not conclusive for cancer,
also known as ASAP; 3) PIN, and 4) cancer. When the diagnosis was adenocarcinoma,
the Gleason score was used for histological differentiation and the tumor
extension was shown by the number of cores positive for tumor and total
percent of tumor in all cores seen.
A subset of 56 patients, from the 376 who
were found to have cancer, underwent radical prostatectomy at our institution.
The pathologic analyses of the prostatectomy specimens were completely
sampled as described previously in detail (14). Organ-confined disease
was defined as tumor that did not extend through the capsule, invade seminal
vesicles, or metastasize to lymph nodes. Gleason score was used for grading.
The tumor volume was determined as a percentage of the prostate gland
involved by carcinoma, as estimated using the grid as described by Humphrey
and Vollmer (15) and extrapolated to cm3 for analysis. Staging
followed the TNM 2002 recommendations (16).
The differences between the pathologic features
were compared between patients whose cancers were detected at a PSA level
between 0 and 4.0 ng/mL and those whose cancers were detected after the
PSA level rose to greater than 4.0 ng/mL. Standard statistics, chi-square
or Fisher’s exact test, and Mann-Whitney test analysis were used
to compare the data.
RESULTS
PSA
was ≤ 4.0 ng/mL in 275 (25.4%) patients, with a mean of 2.85 ng/mL,
(SD 0.94) and median of 3.04 ng/mL (range 0.3 to 4.0 ng/mL). The levels
of PSA were between 0 to 1 ng/mL in 21 (7.6%), 1.1 to 2 ng/mL in 34 (12.4%),
2.1 to 3 ng/mL in 82 (29.8%) and 3.1 to 4 ng/mL in 138 (50.2%) patients.
The remaining 806 (74.6%) had a PSA higher than 4.0 ng/mL, with a mean
of 8.99 ng/mL (SD 9.5 ng/mL), and a median of 6.6 ng/mL, ranging from
4.01 to 146.0 ng/mL.
Patients with PSA ≤ 4 ng/mL were significantly
younger, with a mean age of 59.3 years (p < 0.001), and had lighter
prostate glands 35.0g compared with 51.5g when PSA > 4 ng/mL (p <
0.001) (Table-1).
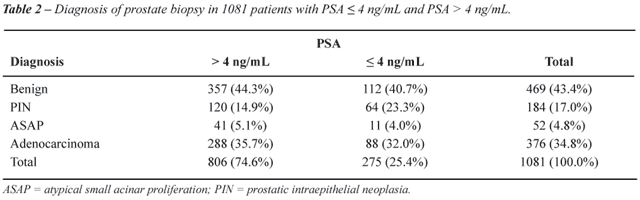
Considering the diagnosis, except for PIN,
that was more frequently diagnosed in men with PSA ≤ 4 ng/mL, there
was no statistical difference between the diagnosis of benign, ASAP and
adenocarcinoma (p = 0.906) (Table-2).
Stratifying PSA levels for men with PSA
≤ 4.0 ng/mL, cancer was diagnosed in 1/21 (4.8%) patients with PSA
level ≤ 1.0 ng/mL, 10/34 (29.4%) with PSA 1.1 to 2 ng/mL, 23/82
(28.0%) with PSA 2.1 to 3 ng/mL and 54/138 (39.1%) with PSA 3.1 to 4 ng/mL.
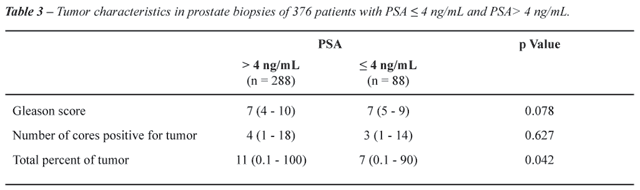
The cancer characteristics were similar
for both groups (Table-3). The median Gleason score was 7 for both (p
= 0.078), the median of number of cores positive for tumor was 4 and 3,
respectively, for PSA > 4 ng/mL and PSA ≤ 4 ng/mL (p = 0.627).
Considering the total percent of tumor involving all cores, patients with
PSA > 4 ng/mL had a median of 11% versus 7% for patients with PSA ≤
4 ng/mL (p = 0.042).
Considering the 71 patients who had information
about the reason of the biopsy, we studied the characteristics of those
33 who were clinical staged T2 comparing with the 38 where digital rectal
examination was normal. Among patients that had abnormalities in the digital
rectal examination, cancer was diagnosed in 13 (39.4%), comparing with
only 8 (21.1%) in 38 without abnormalities in the digital rectal examination
(p < 0.0001). PSA levels were similar for both groups, 2.54 ng/mL for
T2 patients and 2.73 ng/mL for T1, as was the Gleason score, mean 6.7
for the T2 and 6.1 for T1. Tumors were larger for T2 lesions, with mean
number of cores positive for tumor 3.9 and mean total percentage 13.0%,
versus 2.3 cores and 2.7% for T1 lesions.
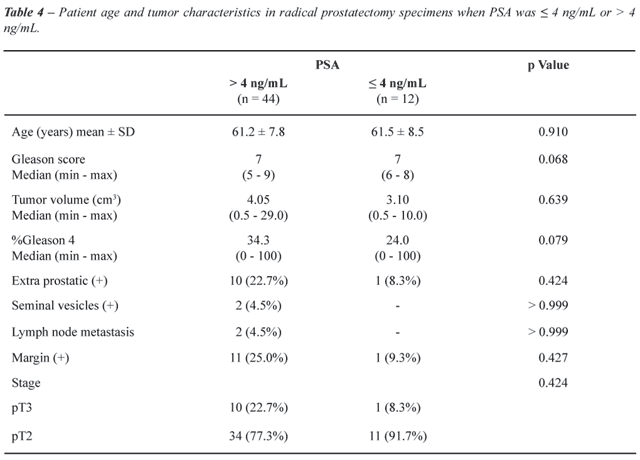
Fifty-six patients underwent radical prostatectomy
and the findings are shown in Table-4. Twelve had PSA ≤ 4 ng/mL.
There was no statistical difference between Gleason score and tumor volume
for both groups of patients. The median Gleason score was 7 for both groups
(p = 0.068), and tumor volume was 10% or 3.1 cm3 and 11% or
4.05 cm3 for ≤ 4 ng/mL and PSA > 4 ng/mL, respectively
(p = 0.689 for percentage and p = 0.639 for cm3). There were
no differences between the groups regarding extra-prostatic extension
(p = 0.424), seminal vesicles infiltration (p > 0.999), lymph node
metastasis (p > 0.999) and positive surgical margins (p = 0.427). One
(8.3%) patient was stage pT3 with PSA ≤ 4 ng/mL and 10 were staged
at this level (22.7%) with PSA > 4 ng/mL (p = 0.424).
In the group of patients with PSA ≤
4 ng/mL there was no insignificant cancer as defined by Epstein et al.
(17) as a tumor volume of less than 0.5 cm3, Gleason score
less than 7, and organ-confined. Additionally, one patient was stage pT3a,
showing extra-prostatic extension and positive surgical margin.
COMMENTS
In
order to minimize economic impact in the health system and maximize the
effectiveness of detecting and treating CaP, various studies have aimed
to find the best levels of PSA and its variations, especially PSA density
and PSA kinetics (18). CaP screening programs have shown that using 4.0
ng/mL as a cutoff results in only clinically significant tumors being
detected, and one third of the men treated for radical prostatectomy disease
that had progressed beyond the prostate (8). Lowering PSA levels to 2.5
ng/mL seems to better detect organ-confined tumors, enhancing chances
of disease-free and overall survival, particularly in younger men (19,20).
In association with lowering PSA levels, the current practice of more
intensive biopsy regimens could lead to the detection of non-significant
tumors. It was the aim of our study to describe histopathological findings
in extended prostate biopsy in patients with PSA levels lower than 4 ng/mL.
We have shown that cancer was diagnosed
in 32% of patients, which is the same proportion of patients diagnosed
with cancer with a PSA > 4 ng/mL, which is the traditional cutoff for
prostate biopsy. We also observed that cancer was diagnosed even in patients
with a very low level of PSA, below 1 ng/mL, and there was significantly
worse disease as PSA levels rose. Malignancy was observed in 29.4%, 28.0%
and 39.1% of patients with PSA levels from 1.1 to 2 ng/mL, 2.1 to 3 ng/mL,
and 3.1 to 4 ng/mL, respectively. Our numbers were even higher than those
reported by the Prostate Cancer Prevention Trial, which indicated that
the overall cancer detection was 15.2%. They found cancer in 6.6% of patients
when PSA was less than 0.5 ng/mL, 10.1% when it was between 0.6 to 1.0
ng/mL, 17.0% from 1.1 to 2 ng/mL, 23.9%, from 2.1 to 3.0 ng/mL, and 26.9%
when PSA was 3.1 to 4 ng/mL (21). The median PSA value for men in their
40s and 50s is approximately 0.7 ng/mL and 0.9 ng/mL, respectively, and
a baseline PSA level greater than the median for each age group was related
to a 12 to 22-fold greater risk of having CaP (22). Although the American
Cancer Society Guidelines recommend screening for CaP before age 50 only
in men with risk factors for CaP, including African-American descent or
a strong family history of CaP, authors have recommended the measurement
of baseline PSA at age 40, which could allow the determination of PSA
kinetics, and is a sensitive marker for prostate cancer diagnosis and
prognostic prediction (22). This knowledge may be changing the standard
practice of urology since in this present study patients with PSA ≤
4 ng/mL were significantly younger. Bill-Axelson et al. (23) have claimed
that initiating screening before age 50 and detecting cancer earlier should
prevent death, especially because patients undergoing radical prostatectomy
younger than 65 years-old have reduced CaP-specific mortality. Sun et
al. (18) have previously shown that in patients younger than 50, PSA levels
of 2.5 ng/mL have specificity of 94% for detecting cancer, and strongly
recommend measuring PSA in younger men. Together with the number of patients
we have just found, biopsy should be recommended when PSA is higher than
the median for that specific age, since almost one third of men will be
diagnosed CaP.
Gleason score is the most important isolated
prognostic factor, and we observed no difference in the Gleason score
between the groups with PSA lower or higher than 4 ng/mL, which both had
a median Gleason score of 7. Furthermore, tumor volume in prostate biopsy
has been addressed as a very important predictor of cancer extension and
outcome. Multiple measurements have been used, including number of positive
cores, total millimeters of cancer amongst all cores, percentage of each
core occupied by cancer and total percent of cancer in the entire specimen.
The best method for determining tumor burden is not yet clear, but estimating
a percentage is easy and has been demonstrated to be a useful predictor
of tumor extension and cancer-free survival rate (24,25). In the current
study we showed no difference considering the number of cores compromised
by tumor, but tumors were smaller when PSA ≤ 4 ng/mL, with a total
percentage of 7% against 11% when PSA > 4 ng/mL. Smaller tumors are
also more likely to be organ-confined. This was not confirmed for patients
undergoing radical prostatectomy where tumor characteristics, including
volume were very similar to those with PSA higher than 4 ng/mL. One explanation
for this fact is a bias considering the choice of treatment. Urologists
could have preferred surgery for those patients with other associated
adverse characteristics leading to similar results, mostly taking into
account tumor volume. This data needs to be clarified in further series.
The detection of organ-confined cancer when
PSA is lower than 4 ng/mL could cause some apprehension in treating “harmless”
or insignificant cancer. Insignificant cancer is defined as tumor with
Gleason pattern less than 4 or 5, organ-confined and volume less than
0.5 cm3 (17). Reports of fewer than 10% of insignificant cancers
have been published, and our series is in agreement with the literature
since we did not find any clinically insignificant cancer. In addition
to the low number of patients who underwent radical prostatectomy with
PSA ≤ 4 ng/mL, our data show tumors that can not be considered insignificant,
with mean Gleason score 6.6, median 7, ranging from 6 to 8. In addition
it is known that presence of tertiary Gleason 4 or 5, and the percent
of a higher Gleason pattern impact the prognosis of prostate cancer. The
mean percent of Gleason pattern 4 for this group of patients was 32%,
which means a 30% reduction in disease free survival in 10 years (26).
Considering tumor volume, McNeal (27) had found good prognosis for tumors
with volume less than 4 cm3. The mean tumor volume of our surgical
specimens was 3.9 cm3, but 33% were higher than 5 cm3,
with one 10 cm3, which could be considered a huge tumor, with
a 33% probability of recurrence in 10 years (26).
One limitation of our study was the lack
of data of PSA velocity (PSAV). PSAV measurement has been shown to be
very helpful, as clinically significant prostate cancer is more likely
to be found in men with a rapidly rising PSA. Studies suggest that for
men with a total PSA higher than 4 ng/mL, a PSA velocity of 0.75 ng/mL/year
is an indication for biopsy. However, in men whose total PSA level is
lower than 4 ng/mL, an ideal cutoff has not yet been determined and should
range from 0.1 to 0.5 ng/mL/year (28-30). It has been demonstrated that
for each 0.1 ng/mL per year increase in PSA, the likelihood of death from
prostate cancer increases 15%. For men with a consistent increase in PSA
of 0.35 ng/mL per year or higher, the relative risk of dying of prostate
cancer is 5 times higher in the next 2 to 3 decades than for men with
lower PSA increases(31). Nevertheless, this weak point may be overcome
by the findings recently published by Yu X et al. (32). These authors
have shown a correlation between total PSA and PSAV, describing a PSAV
of more than 2 ng/mL per year in only 1% and 14% of patients whose PSA
total levels were lower than 2.5 ng/mL or between 2.5 ng/mL and 4 ng/mL,
respectively, indicating a less aggressive and more curable disease.
In conclusion, our findings show that in
the extended biopsy era cancer will be detected in 32% of patients when
biopsy is performed with PSA below 4 ng/mL. Gleason score and number of
cores positive for cancer are similar to those with PSA > 4 ng/mL.
Although cancer characteristics in radical prostatectomy were comparable
for both groups as Gleason score, percentage of Gleason pattern 4, tumor
volume and staging, patients that undergo biopsy with PSA lower than 4
ng/mL are younger and have smaller tumors in biopsies as measured by the
total percent of tumor, and, consequently have better chances of having
less aggressive tumors. Because of the small number of patients submitted
to radical prostatectomy with PSA ≤ 4 ng/mL, other studies, with
larger series are warranted to confirm our findings.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Amling CL, Blute ML, Lerner SE, Bergstralh EJ, Bostwick DG, Zincke H: Influence of prostate-specific antigen testing on the spectrum of patients with prostate cancer undergoing radical prostatectomy at a large referral practice. Mayo Clin Proc. 1998; 73: 401-6.
- D’Amico AV, Whittington R, Malkowicz SB, Fondurulia J, Chen MH, Tomaszewski JE, et al.: The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998; 160: 2096-101.
- Stephenson RA: Population-based prostate cancer trends in the PSA era data from the Surveillance, Epidemiology, and End Results (SEER) Program. Monogr Urol. 1998; 19: 3-19.
- Newcomer LM, Stanford JL, Blumenstein BA, Brawer MK: Temporal trends in rates of prostate cancer: declining incidence of advanced stage disease, 1974 to 1994. J Urol. 1997; 158: 1427-30.
- Catalona WJ, Loeb S: The PSA era is not over for prostate cancer. Eur Urol. 2005; 48: 541-5.
- Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC: Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001; 166: 416-9.
- Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, et al.: Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991; 324: 1156-61. Erratum in: N Engl J Med 1991; 325: 1324.
- Catalona WJ, Smith DS, Ratliff TL, Basler JW: Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993; 270: 948-54.
- Catalona WJ, Ramos CG, Carvalhal GF, Yan Y: Lowering PSA cutoffs to enhance detection of curable prostate cancer. Urology. 2000; 55: 791-5.
- Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y, et al.: Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002; 60: 469-73; discussion 473-4.
- Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaeker RF, Kranse R: Prostate cancer detection at low prostate specific antigen. J Urol. 2000; 163: 806-12.
- Zhu H, Roehl KA, Antenor JA, Catalona WJ: Biopsy of men with PSA level of 2.6 to 4.0 ng/mL associated with favorable pathologic features and PSA progression rate: a preliminary analysis. Urology. 2005; 66: 547-51.
- Siu W, Dunn RL, Shah RB, Wei JT: Use of extended pattern technique for initial prostate biopsy. J Urol. 2005; 174: 505-9.
- Epstein JI, Amin M, Boccon-Gibod L, Egevad L, Humphrey PA, Mikuz G, et al.: Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl. 2005; 216: 34-63.
- Humphrey PA, Vollmer RT: Intraglandular tumor extent and prognosis in prostatic carcinoma: application of a grid method to prostatectomy specimens. Hum Pathol. 1990; 21: 799-804.
- Greene FL, Page DL, Fleming ID: AJCC Cancer Staging Manual. 6th edition. New York, Springer; 2002.
- Epstein JI, Walsh PC, Carmichael M, Brendler CB: Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994; 271: 368-74.
- Sun L, Moul JW, Hotaling JM, Rampersaud E, Dahm P, Robertson C, et al.: Prostate-specific antigen (PSA) and PSA velocity for prostate cancer detection in men aged <50 years. BJU Int. 2007; 99: 753-7.
- Dall’Oglio MF, Crippa A, Passerotti CC, Nesrallah LJ, Leite KR, Srougi M: Serum PSA and cure perspective for prostate cancer in males with nonpalpable tumor. Int Braz J Urol. 2005; 31: 437-44.
- Catalona WJ, Ramos CG, Carvalhal GF, Yan Y: Lowering PSA cutoffs to enhance detection of curable prostate cancer. Urology. 2000; 55: 791-5.
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al.: Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004; 350: 2239-46. Erratum in: N Engl J Med. 2004; 351: 1470.
- Loeb S, Roehl KA, Antenor JA, Catalona WJ, Suarez BK, Nadler RB: Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006; 67: 316-20.
- Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, et al.: Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005; 352: 1977-84.
- Rubin MA, Bassily N, Sanda M, Montie J, Strawderman MS, Wojno K: Relationship and significance of greatest percentage of tumor and perineural invasion on needle biopsy in prostatic adenocarcinoma. Am J Surg Pathol. 2000; 24: 183-9.
- Freedland SJ, Csathy GS, Dorey F, Aronson WJ: Clinical utility of percent prostate needle biopsy tissue with cancer cutpoints to risk stratify patients before radical prostatectomy. Urology. 2002; 60: 84-8.
- Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM: Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999; 281: 1395-400.
- McNeal JE: Prostate cancer volume. Am J Surg Pathol. 1997; 21: 1392-3.
- Fang J, Metter EJ, Landis P, Carter HB: PSA velocity for assessing prostate cancer risk in men with PSA levels between 2.0 and 4.0 ng/ml. Urology. 2002; 59: 889-93; discussion 893-4.
- Berger AP, Diebl M, Steiner H, Bektic J, Pelzer A, Leonhartsberger N et al., Longitudinal PSA changes in men with and without prostate cancer: assessment of prostate cancer risk. J Urol. 2005; 173 (Suppl. 4): 402.
- Catalona WJ, Loeb S: The PSA era is not over for prostate cancer. Eur Urol. 2005; 48: 541-5.
- Carter HB, Ferrucci L, Kettermann A, Landis P, Wright EJ, Epstein JI, et al.: Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006; 98: 1521-7.
- Yu X, Loeb S, Roehl KA, Han M, Catalona WJ: The association between total prostate specific antigen concentration and prostate specific antigen velocity. J Urol. 2007; 177: 1298-302; discussion 1301-2.
____________________
Accepted after revision:
February 12, 2008
_______________________
Correspondence address:
Dr. Katia R. M. Leite
Rua Adma Jafet, 91
Sao Paulo, SP, 01308-050, Brazil
Fax: +55 11 3231-2249
E-mail: katiaramos@uol.com.br
EDITORIAL COMMENT
This is a well conducted study concluding that when PSA is below 4 ng/mL, cancer is detected in a proportion equal to the proportion diagnosed with a PSA > 4 ng/mL, and tumor characteristics are similar between the two groups. These findings are supported by other studies. Krumholtz et al. (1) evaluated the pathologic characteristics of clinical stage T1c prostate cancers detected in the 2.6 to 4.0 ng/mL PSA range and compared them with cancers concurrently detected in the 4.1 to 10.0 ng/mL. The authors found that men detected at the 2.6 to 4.0 ng/mL PSA range had significantly smaller cancer volumes however, no difference was found in the proportion of tumors that met previously published criteria of “clinically insignificant” (organ confined, less than 0.2 cm3 tumor volume, and Gleason sum 6 or less) or “clinically unimportant” (organ confined, less than 0.5 cm3 tumor volume, and Gleason sum 6 or less) tumors. Using the lower PSA cutoff point resulted in the detection of a significantly higher percentage of organ-confined tumors. The authors conclude that the use of a 2.6 ng/mL PSA threshold for screening resulted in the more frequent detection of small, organ-confined tumors without over detecting possibly clinically insignificant ones. Obviously, additional studies in larger populations with longer follow-up are needed to confirm these findings.
REFERENCE
- Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y, et al.: Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002; 60: 469-73; discussion 473-4.
Dr.
Athanase Billis
Full-Professor of Pathology
State University of Campinas, Unicamp
Campinas, Sao Paulo, Brazil
E-mail: athanase@fcm.unicamp.br
EDITORIAL COMMENT
Early
in the PSA era patients with a serum prostate-specific antigen (PSA) level
> 4.0 ng/mL and a normal digital rectal examination (DRE) were recommended
to undergo prostate biopsy because of a 20-30% risk of prostate cancer
at a pre-specified sensitivity of 95%. The majority of such patients have
clinically important cancers and the rate of indolent disease, defined
as specimen Gleason score 2-6, no extra-prostatic extension, and no Gleason
pattern 4/5 is generally < 20%. Many have argued that a PSA threshold
for biopsy of 4.0 is more frequently associated with under- rather than
over-diagnosis as rates of non-organ-confined cancer (25-35%) are 2 to
4 times higher than indolent cancers, whereas cancers detected in the
2.6-4.0 PSA range are more likely to be organ-confined without substantial
differences in the rate of low-grade or indolent cancer. In a longitudinal
screening study, a decreased risk of PSA-defined biochemical recurrence
was observed for patients treated by radical prostatectomy after lowering
the PSA threshold for biopsy from 4.0 to 2.5 (1). As such, a lowering
of the PSA level for biopsy to 2.5 has been advocated to increase the
detection of clinically significant cancers at a more curable stage, and
this had been adopted in the guidelines of some professional societies
(2).
The Prostate Cancer Prevention Trial (PCPT)
has challenged the validity of any PSA threshold for biopsy as no specific
PSA value had sufficient sensitivity and specificity for the detection
of prostate cancer to be clinically useful (3). Based on the results of
patients who had an end-of-study biopsy without usual clinical implications,
there was a continuum of cancer risk at all PSA values. Among patients
with a PSA < 1.0, 1.1-2.0, and 2.1-3.0, the cancer detection rate was
9%, 17%, and 24%, respectively and the corresponding proportion of cancers
graded as Gleason 7-10 was 11%, 12% and 19% (4). These results indicate
that there is no PSA below which the risk of having cancer is zero.
In the current study, Leite et al. report
on the biopsy and pathological characteristics of a cohort of patients
biopsied with a PSA < 4. Reasons for biopsy included abnormal DRE,
prior biopsies showing atypical small acinar proliferation or prostatic
intraepithelial neoplasia, persistently elevated PSA and negative prior
biopsy, or a positive family history, so that the population studied is
not fully representative of the general population usually subjected to
opportunistic screening. Nonetheless the findings are illuminating, demonstrating
similar rates of prostate cancer in those with a PSA < 4 vs. > 4
(32 vs. 36%), no difference in tumor grade (median score 7 in both groups),
and slightly fewer positive cores in the PSA < 4 group. In the small
subset of patients who underwent radical prostatectomy, there was no difference
in tumor volume, grade, or pathological stage. Surprisingly, and unlike
our own experience with similar patients where the incidence of indolent
cancers is higher in men with PSA < 4, the authors found no indolent
cancers as defined by Epstein’s criteria of organ-confined tumors
of volume < 0.5 cc and grade < 7. This likely reflects the indications
for biopsy in this population and less widespread and repeated screening
than in the United States.
What then is the optimal PSA cutoff for
recommending biopsy in 2008? The theoretical answer is that the optimal
threshold is one that maximizes detection of biologically significant
but curable cancers, reduces prostate-cancer-specific mortality, and minimizes
over-diagnosis of indolent disease. The practical answer is one that recognizes
that PSA represents a continuum of risk that is also impacted by many
other factors, and that the best way decides whom to biopsy includes a
consideration of all of the relevant factors. At the Cleveland Clinic,
we have stopped reporting a “normal” cutoff for PSA on our
lab reports and substituted the following: “Published data from
the Prostate Cancer Prevention Trial demonstrated that there is no PSA
level below which the risk of having prostate cancer is zero. For an individual
patient, the significance of a PSA level should be interpreted in a broad
clinical context, including age, race, family history, digital rectal
exam, prostate size, results of prior prostate biopsy, and use of 5 alpha
reductase inhibitors. Considering the high incidence of asymptomatic cancer
in the general population that may not pose an ultimate risk to the patient,
the decision to recommend urological evaluation or prostate biopsy should
be individualized after considering all of these factors.” We have
encouraged the use of the PCPT risk calculator (available at www.compass.fhcrc.org/edrnnci/bin/calculator/main.asp)
as one tool (validated published nomograms for this purpose also exist)
to achieve the goal of defining individual risk prior to recommending
biopsy. Using this calculator, a 55 year old Caucasian male with a negative
DRE, a PSA of 1.5, and no family history of prostate cancer has a 19%
risk of having prostate cancer but only a 2% risk of having high grade
(Gleason 7 or greater) disease, information that can give the patient
a more precise estimate of the risks and benefits of undergoing biopsy
before deciding whether to have it done. For a 55 year old African American
man with a normal DRE, a positive family history, and PSA of 2.4, the
calculator estimates a risk of any cancer at 31% and of high grade cancer
at 8%; here the risk: benefit ratio probably justifies biopsy even though
his PSA is generally considered below the current threshold. Adoption
of this approach outside of the U.S. requires construction and validation
of similar models on local populations; ultimately, proof of the utility
of PSA screening at all awaits the reporting of large screening trials
(the ERSPC and PLCO) currently nearing completion.
REFERENCES
- Jang TL, Han M, Roil KA, Hawkins SA, Catalona WJ: More favorable tumor features and progression-free survival rates in a longitudinal prostate cancer screening study: PSA era and threshold-specific effects. Urology. 2006; 67: 343-8.
- Smith RA, Cokkinides V, Eyre HJ: American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006; 56: 11-25; quiz 49-50.
- Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, et al.: Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005; 294: 66-70.
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al.: Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004; 350: 2239-46. Erratum in: N Engl J Med. 2004; 351: 1470.
Dr.
Andrew J. Stephenson
Dr. J. Stephen Jones
Dr. Eric A. Klein
Section of Urologic Oncology
Glickman Urologic and Kidney Institute
Cleveland Clinic, Ohio, USA
E-mail: kleine@ccf.org