BOTULINUM
TOXIN TREATMENT OF URETHRAL AND BLADDER DYSFUNCTION
(
Download pdf )
CHRISTOPHER P. SMITH, GEORGE T. SOMOGYI, MICHAEL B. CHANCELLOR
Departments of Urology and Pharmacology, School of Medicine, University of Pittsburgh, Pittsburgh, Philadelphia, USA
ABSTRACT
There has been tremendous excitement with the use of botulinum toxin for the treatment of various urethral and bladder dysfunction over the past several years. Botulinum toxin is the most lethal naturally occurring toxin known to humankind. Why, then, would an urologist want to use this agent to poison the bladder or urethral sphincter? In this article, we will review the mechanisms underlying the effects of botulinum toxin treatment. We will discuss the current usage of this agent within the urologic community and will provide perspectives on future targets of botulinum toxin.
Key words:
botulinum toxins; bladder, neurogenic; urethra
Int Braz J Urol. 2002; 28: 545-52
HISTORY OF BOTULINUM TOXIN’S MEDICAL DEVELOPMENT
The world’s most potent biological toxin, botulinum toxin, was first isolated more than 100 years ago by van Ermengem in 1897 (10). The toxin acts by inhibiting acetylcholine release at the presynaptic cholinergic junction. Starting in the late 1980’s, the urologic community has explored the use of botulinum toxin type A (BTX-A) to treat spinal cord injured patients who suffer from detrusor external sphincter dyssynergia (DESD) (2-4). A resurgent of interest over the past 5 years was lead by Schurch and colleagues, who reported successful treatment of spinal cord injured patients with detrusor hyperreflexia using intravesical BTX-A injections at multiple sites (5).
WHAT IS THE STORY OF HOW THIS "FOOD POISON" BECAME A USEFUL MEDICAL DRUG?
Botulinum poisoning was first described in cases of sausage poisoning in the late 1700’s in Germany. A local medical officer collected data on 230 cases of botulism and the illness became known as “Kerner’s disease” (6). It was not until 1897 that van Ermengem isolated the spore-forming obligate anaerobic bacteria, Clostridium botulinum (1).
HOW DOES BOTULINUM TOXIN CAUSE PARALYSIS?
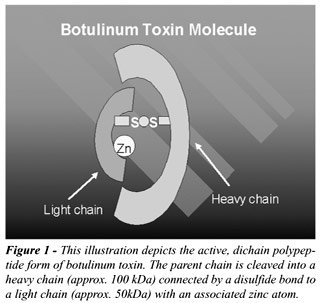
Botulinum
toxins are synthesized as single chain polypeptides with a molecular weight
of around 150 kilo Daltons (kDa) (7). Initially, the parent chain is cleaved
into its active, dichain polypeptide form, consisting of a heavy chain
(approx. 100 kDa) connected by a disulfide bond to a light chain (approx.
50kDa), with an associated zinc atom (Figure-1) (8). Three steps are required
for toxin induced paralysis: 1)- binding and internalization of the toxin
within the nerve terminal; 2)- translocation of the light-chain into the
cytosol; and 3)- inhibition of neurotransmitter release.
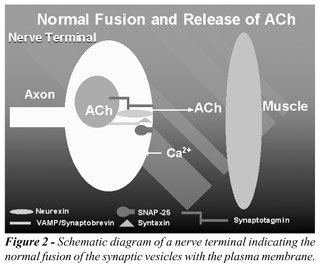
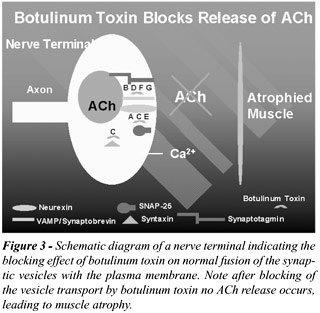
Acetylcholine release involves the ATP-dependent
transport of the vesicle from the cytosol to the plasma membrane (9).
Vesicle docking requires the interaction of various cytoplasmic, vesicle,
and target membrane proteins, some of which are specifically targeted
with clostridial neurotoxins. BTX-A, for example, cleaves the cytosolic
translocation protein SNAP-25, thus preventing vesicle fusion with the
plasma membrane (Figures-2 and 3) (10).
DIFFERENT APPLICATIONS OF BOTULINUM TOXIN
Seven immunologically distinct neurotoxins types are known, and they are typically labeled from A-G. BTX-A (Botox®, Allergan) received FDA approval in 1989 for the treatment of strabismus, benign essential blepharospasm and disorders of the VIIth nerve. Since its introduction into clinical use in the 1980’s, BTX-A has been successfully used to treat various conditions including blepharospasm, strabismus, focal dystonias, muscle spasms and spasticity, axillary hyperhidrosis, and achalasia (11-15). More recently, the U.S. FDA approved a BTX-B complex preparation (Myobloc™, Elan) for clinical use in cervical dystonia patients.
BOTULINUM TOXIN’S UROLOGIC APPLICATIONS
Sphincter
Application
Urological applications of BTX-A have been
primarily associated with cases of Detrusor External Sphincter Dyssynergia
(DESD). Management of Spinal Cord Injured (SCI) patients was revolutionized
with the development of clean intermittent catheterization (CIC) by Lapides
in 1971 (16). However, not all patients are capable of performing CIC,
and require an alternative that decreases outlet resistance and allows
continuous bladder decompression. Various alternatives have been described,
including external sphincterotomy, radical transurethral resection of
the prostate (TURP), and various denervation procedures, i.e., dorsal
rhizotomy (17). These procedures are unfortunately permanent and irreversible,
and carry with them inherent risks (i.e., bleeding, stricture formation,
fistulas).
BTX-A represents a viable option in the
treatment of DESD. The toxin acts at the neuromuscular junction of the
external sphincter to block vesicle transport of acetylcholine; in essence,
producing a chemical denervation. The clinical effects begin within 2-3
days and are reversible as terminal nerve sprouting occurs within 3-6
months (18). Injection of BoNT/A (i.e., BoNT = laboratory grade botulinum
toxin) into the sternomastoid muscle of mice has been shown to induce
the formation of terminal nerve sprouts from the parent terminal (19).
The sprouts form functional synapses with the muscle, but eventually regress
at a time when the parent nerve terminal regains the ability to release
neurotransmitters. It remains to be seen whether similar processes occur
in autonomic nerves innervating the lower urinary tract.
Dykstra investigated the effects of BTX-A
injection in two studies of SCI patients with DESD. In the first study,
published in 1988, all 10 patients that were evaluated by electromyography
after injection showed signs of sphincter denervation (4). Urethral pressure
profile decreased by an average of 27cm/H2O , and post-void residuals
decreased by an average of 146cc after toxin injection. In 1990, Dykstra
published the only double-blind placebo controlled study of BTX-A injection
into the external urethral sphincter of 5 men with SCI and DESD (3). Electromyography
of the external urethral sphincter indicated denervation in the 3 patients
who received toxin injections. The urethral pressure profile decreased
an average of 25cm/H2O, post-void residual decreased an average of 125mL
and bladder pressure during voiding decreased to an average of 30cm/H2O.
Parameters were unchanged from baseline in the 2 patients who received
normal saline injections.
We performed a prospective study on 21 patients
referred to our clinic with voiding dysfunction (20). All patients were
evaluated with video-urodynamics. Follow-up ranged from 3-16 months. Following
urethral injection of Botox®, voiding pressures decreased an average
of 38%. Sixty-seven percent of patients reported improvement in voiding
patterns. No complications or side effects were noted. Our results are
consistent with the largest series to date treating DESD with BTX-A; in
this study, Schurch treated 24 patients with SCI and DESD with BTX-A injection
(21). Significant improvement in DESD was noted in 21/24 pts (88%) with
decreased post-void residuals in most patients. The effects lasted 3-9
months, with no adverse events reported. Thus, BTX-A toxin injections
are a safe and efficacious treatment option for DESD.
The clinical success of BTX-A is supported
by laboratory research demonstrating marked decreases in the release of
labeled norepinephrine and acetylcholine in BoNT/A injected rat urethral
sphincters (22). While the therapeutic effect of inhibiting acetylcholine
release is obvious, blockage of norepinephrine release may provide clinical
benefit by inhibiting sympathetic transmission and smooth muscle dyssynergia.
In addition to classic neuropathic DESD,
we have expanded the indications for use of botulinum A toxin to include
patients with a variety of bladder outlet obstructions, excluding those
patients with obstruction secondary to fibrosis. We have successfully
used botulinum A toxin to treat voiding dysfunction in multiple sclerosis
patients with DESD, patients with pelvic floor spasticity, and even in
an acontractile multiple sclerosis patient who wished to void by Valsalva
(20). Recently, we reported a case of functional urethral obstruction
and detrusor acontractility following pubovaginal sling surgery that was
successfully treated by botulinum A toxin urethral sphincter injection
(23).
We perform Botox® urethral sphincter
injections by mixing one vial (100 units) of Botox® with 10cc of saline
just prior to injection. It is important not to shake vial as this may
break the disulfide linkage between the light and heavy chains and render
the toxin ineffective. Using a collagen injection needle, (we prefer Cook®
because of the sharper end) injections of 2.5cc each are made at the 12,
3, 6, and 9 o’clock positions at the level of the striated sphincter.
Injections must be directed deeper than collagen injections in order to
target nerve terminals innervating skeletal muscle. We also flush the
needle with 0.2cc of saline at the end of the procedure to ensure that
no toxin is wasted.
Bladder
Application
Data has been accumulating on the clinical
application of BTX-A to detrusor muscle in hyperreflexic bladders of spinal
cord injured patients. A preliminary study by Schurch and colleagues in
31 patients with detrusor hyperreflexia demonstrated a significant increase
in mean maximum bladder capacity (296mL to 480mL, p<0.016), and a significant
decrease in mean maximum detrusor voiding pressure (65 to 35cm H2O, p<0.016)
in patients injected with BTX-A (5). A follow-up long-term study completed
by the same investigators in 87 patients with detrusor hyperreflexia corroborated
the efficacy of intravesical botulinum toxin injection presented in their
earlier work (24). In addition, they reported clinical responses lasted
4-14 months, and observed no adverse effects with treatment. Detrusor
muscle injections were performed in over 30 sites, with either 300 units
of Botox® or 500-750 units of Dysport®. The trigone was spared,
presumably, to avoid the potential complication of vesicoureteral reflux.
In contrast, Del Popolo noted hyposthenia
in 5/61 patients treated with high-dose intravesical BTX-A injections
(300u of Botox® or 1,000u Dysport®) (25). The supralesional weakness
was transient in nature, disappearing 2-4 weeks after injection, and was
abolished with lower dosage injections (500u Dysport®). Clearly, the
dose and the volume injected appear to play a significant role in inducing
systemic toxicity with BTX-A. Multiple injections of lower doses would
be expected to have a more localized and less systemic effect. However,
the main disadvantage of intravesical BTX-A injections for many urologists
is the repeated cystoscopies and toxin injections that are necessary to
maintain clinical results.
BTX-A injections have extended beyond the
realm of neurogenic bladders to patients with non-neurogenic voiding and
storage disorders. Radziszewski and associates reported favorably on the
effects of intravesical BTX-A injections in a pilot study of patients
with either idiopathic bladder overactivity or functional outlet obstruction
(26). Following intravesical or sphincteric BTX-A injections, patients
demonstrated resolution of incontinence and improved voiding efficiency,
respectively. Finally, Zermann and colleagues presented their experience
with intravesical BTX-A injection in 7 patients with severe urgency-frequency
syndrome refractory to anticholinergic therapy or electrical stimulation
(27). In contrast to other studies involving intravesical injections of
BTX-A, the authors targeted the trigone and bladder base with 5-7 injections
of 50, 100 or 200 units of Botox®. Four of 7 patients responded to
treatment with decreases in frequency and increased bladder capacity.
No mention is made of vesicoureteral reflux as a complication of treatment.
We recently presented a single surgeon’s
experience using Botox® in the bladder and urethra of 50 patients
for a variety of dysfunctions over the past 3 years (28). Between October
of 1998 and October 2001, 50 patients (age range 31-84) were injected
with botulinum toxin into the bladder (n=10) or urethra (n=40). Of these,
19 were men and 31 were women. Voiding dysfunctions were a result of both
neurogenic and non-neurogenic conditions and included: multiple sclerosis,
spinal cord injury, cerebral vascular accident, overactive bladder, interstitial
cystitis, and dysfunctional voiding. Procedures were performed using light
sedation. Patients were treated with either 100 units of Botox® divided
in equal doses into the 4 quadrants of the external sphincter, or via
injection into the bladder base using 100-300 units of botulinum toxin
diluted in 20mL of sterile saline. Presently, 15 of these patients have
undergone further injections (as many as 4) at intervals of 6 months or
more. Maximal efficacy of botulinum injection was achieved within 7 days
post injection. Analysis of the 50 patients indicates that 41 of 50 patients
(82%) report a decrease or absence of incontinence as well as a significant
decrease in voiding symptoms. Sleep quantity and quality increased in
more than 50% of patients. Follow-up of these patients indicate that effects
lasted up to 12 months. No patient developed stress incontinence or urinary
retention.
These latest clinical findings are supported
by research of ours and others demonstrating the efficacy of BoNT’s
on autonomic nerves (29-32). Our studies found significant decreases in
the release of labeled norepinephrine and acetylcholine in BoNT/A injected
rat bladders (32).
RESEARCH DEVELOPMENT
Botulinum
Toxin Isoforms
An interesting side effect of patients with
cervical dystonia injected with BTX-B (Myobloc™, Elan) was the development
of dry mouth (33). A rare occurrence following BTX-A treatment, dry mouth
was unexpected because the salivary glands were farther from the injection
site than relatively unaffected lingual or lower facial muscles. This
implies that BTX-B may have a greater affinity for cholinergic nerves
innervating the salivary gland rather than lingual or lower facial muscles
or, alternatively, that there are a higher number of BTX-B receptors in
salivary gland compared to muscles of the lower face and tongue. Future
studies should clarify whether similar effects are seen in parasympathetic
cholinergic nerves innervating the lower urinary tract.
In addition, evidence from Carpenter’s
experiments in the late 1960’s, as well as our labs’, suggests
that rat bladders are significantly more sensitive to the effects of BoNT/D
than BoNT/A (29,34). In fact, Carpenter found that parasympathetic blockade
with BoNT/D occurred before somatic neuromuscular blockade. It remains
to be seen whether these effects are merely due to differing sensitivities
of various cholinergic nerve endings to different toxins, or whether BoNT/D’s
greater efficacy in the bladder is due to an effect on non-cholinergic
transmission. Currently, no data exists on whether these same differences
in rat bladder sensitivity to toxin isoforms exist in the human bladder.
Afferent
Nerve Effects
Several investigators have demonstrated
in vitro evidence of an afferent effect of botulinum toxin. Welch and
colleagues reported that neuropeptide release from rat dorsal root ganglia
was inhibited by botulinum toxin (BoNT/A, B, C1, F) treatment, while Purkiss
and colleagues noted that incubation of rat dorsal root ganglia with BoNT/A
inhibited release of radioactively labeled glutamate (35,36). The inhibition
of transmitter release from nociceptive neurons could impair mechanisms
involved with central sensitization, and place botulinum toxin as a therapeutic
agent for conditions such as chronic pain.
Current in vivo studies support a role for
BTX-A in relieving nociceptive pain. In a model of pain associated with
formalin-induced inflammation, rats were pretreated in the hind paw with
BTX-A prior to injection with formalin (37). Formalin provokes pain via
direct stimulation of nociceptors (Phase 1) and, subsequently, by inflammation
(Phase II). Formalin was injected 5 and 12 days after BTX-A injection.
Surrogate markers of pain included paw-licking and paw-lifting behavior.
Pretreatment with BTX-A significantly reduced pain at 5 and 12 days post-injection.
These results support clinical observations that BTX-A has an antinociceptive
effect that is independent of its effects on the neuromuscular junction.
We have preliminary results suggesting that
BoNT/A treatment inhibits afferent nerve mediated bladder strip contractions,
presumably by blocking neurotransmitter release from peripheral afferent
nerve terminals in the bladder (38). BoNT/A treatment significantly decreased
afferent nerve mediated contractions to both electrical and chemical stimulation,
by 44.6% and 35.1%, respectively, compared to saline treated animals (p<0.05).
In addition, we have clinical experience
with Botox® treatment in a 42 year-old female patient suffering from
recalcitrant interstitial cystitis (IC) (personal observation). Under
light sedation, following hydrodistension with saline (80cm) for 5 minutes,
100 units of Botox®, diluted in 100mLmL of saline, was instilled in
the bladder and held for 30 minutes. The patient was discharged home the
same day and followed up over the ensuing 6 months. One week following
Botox® treatment, the patient noted marked improvement in her voiding
symptoms, characterized by decreased frequency, urgency, and urge incontinent
episodes. Nocturia decreased 4-fold, and painful bladder symptoms diminished
greatly as evidenced by a 50% decrease in oral pain medication usage.
On a visual analog scale, patients bother score decreased from a 10 to
a 5 following BTX-A treatment. Maximal therapeutic effects lasted 3 months,
with some improvement still noted at 6 months post-treatment. Our preliminary
findings may lead to new therapeutic applications of BTX-A, such as treating
conditions associated with increased afferent nerve excitability (i.e.,
spinal cord injury, chronic inflammation).
Clearly, BTX-A has a much wider spectrum
of application within the urologic field than merely the treatment of
DH and DESD in SCI patients. Treatment should be extended to other fields
including the MS population and non-neurogenic voiding and storage disorders.
Our basic research evidences that BoNT/A inhibits norepinephrine release
in the rat bladder and urethra should prompt studies investigating the
effects of botulinum toxin on disorders of increased sympathetic activity
(e.g. functional bladder neck obstruction, detrusor internal sphincter
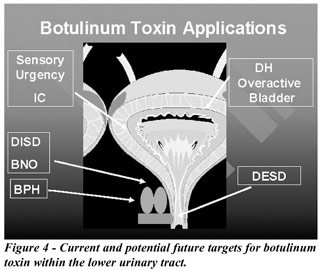
dyssynergia and BPH). Finally, if afferent nerve transmission is impaired
by botulinum toxin, a significant patient population will be opened to
this treatment (Figure-4).
CONCLUSIONS
Since the 1980’s, injection of botulinum toxin has proven to be a safe and effective therapy for a variety of somatic and autonomic motor disorders. Urologists are now finding clinical success with urethral and bladder BTX-A injections in the treatment of detrusor-sphincter dyssynergia, non-neurogenic pelvic floor spasticity, and refractory overactive bladder. Many interesting research questions remain regarding BTX’s effect on the neural pathways of the lower urinary tract (39). However, one cannot deny the ingenuity of man in transforming the lethal toxin of Clostridium botulinum into a modern day therapeutic medicine.
REFERENCES
- Van Ermengem E: Ueber einen neuen anaeroben Bacillus and seine Beziehungen zum Botulisms. Ztsch Hyg Infekt. 1897; 26: 1.
- Petit H, Wiart E, Gaujard E, LeBreton F, Ferriere JM, Lagueny A, et al.: Botulinum A toxin treatment for detrusor-sphincter dyssynergia in spinal cord disease. Spinal Cord. 1998; 36: 91.
- Dykstra DD, Sidi AA, Scott AB, Pagel JM, Goldish GD: Effects of botulinum A toxin on detrusor-sphincter dyssyngeria in spinal cord injury patients. J Urol. 1988; 139: 919.
- Dykstra DD, Sidi A: Treatment of detrusor-sphincter dyssyngeria with botulinum A toxin: a double blind study. Arch. Phys. Med. Rehabil. 1990; 71: 24.
- Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D: Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000; 164: 692-7.
- Dickson EC: Botulism. A clinical and experimental study. Rockefeller Inst Med Res Mong. 1918; 8: 1.
- Dolly JO: General properties and cellular mechanisms of neurotoxins, in Jankovic, J. and Hallet, M. (eds): Therapy with Botulinum Toxin. New York, Marcel Dekker. 1994.
- Simpson LL: Peripheral actions of the botulinum toxins, in Simpson, L.L. (ed): Botulinum Neurotoxin and Tetanus Toxin. New York, Academic Press. 1989; pp153-178.
- Barinaga M: Secrets of secretion revealed. Science. 1993; 260: 487.
- Schiavo G, Santucci A, DasGupta BR, et al: Botulinum neurotoxins serotypes A and E cleave Snap-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993; 335: 99.
- Grazko MA, Polo KB, Jabbari B: Botulinum toxin A for spasticity, muscle spasms, and rigidity. Neurology. 1995; 45: 712.
- Jankovic J, Schwartz K, Donovan DT: Botulinum toxin in the treatment of cranial-cervical dystonias and hemifacial spasm. J. Neurol. Neurosurg. Psychiatry. 1990; 53: 633.
- Scott AB: Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981; 79: 734.
- Kolbasnik J, Waterfall WE, Fachnie B, Chen Y, Tougas G: Long-term efficacy of Botulinum toxin in classical achalasia: a prospective study. Am J Gastroent. 1999; 94(12): 3434-9.
- Schnider P, Binder M, Kittler H, Birner P, Starkel D, Wolff K, et al.: A randomized, double-blind, placebo-controlled trial of botulinum A toxin for severe axillary hyperhidrosis. Brit J Dermat. 1999; 140: 677-80.
- Lapides J, Diokno AC, Silber SJ, Lowe BS: Clean, intermittent self-catheterization in the treatment of urinary tract disease. Trans Am Assoc Genitourin Surg. 1971; 63: 92-6.
- Koyanagi T, Morita H, Takamatsu T, Taniguchi K, Shinno Y: Radical transurethral resection of the prostate in male paraplegics revisited: further clinical experience and urodynamic considerations for its effectiveness. J Urol. 1987; 137: 72.
- Borodic GE, Joseph M, Fay L, Cozzolino D, Ferrante RJ: Botulinum A toxin for the treatmnent of spasmodic torticollis: dysphagia and regional toxin spread. Head Neck. 1990; 12: 392.
- de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO: Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci. 1999; 96: 3200-5.
- Phelan MW, Franks M, Somogyi GT et al.: Botulinum toxin urethral sphincter injection to restore bladder emptying in men and women with voiding dysfunction. J Urol. 2001; 165: 1107-10.
- Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB: Botulinum A toxin as a treatment of detrusor-sphincter dyssyngeria; a prospective study in 24 spinal cord injury patients. J Urol. 1996; 155: 1023.
- McNeil BK, Smith CP, Franks ME, Ghosh R, de Groat WC, Chancellor MB, et al.: Effect of botulinum toxin A on urethral neurotransmitter release: Implications on somatic/autonomic nerve transmission. J Urol. 2001; 165: 277, Abstract.
- Smith CP, O’Leary M, Erickson J, Somogyi GT, Chancellor MB: Botulinum toxin urethral sphincter injection resolves urinary retention after pubovaginal sling operation. Int Urogynecology J and Pelvic Floor Dysfunction. 2002; 13: 55-6.
- Schurch B, Stöhrer M, Kramer G, Grosse J, Schmid D, Hauri D: Botulinum Toxin-A to treat detrusor hyperreflexia in spinal cord injured patients. Neurourology & Urodynamics. 2001; 20: 521-2, Abstract.
- Del Popolo G: Botulinum-A toxin in the treatment of detrusor hyperreflexia Neurourology & Urodynamics. 2001; 20: 522-4, Abstract.
- Radziszewski P, Dobronski P, Borkowski A: Treatment of the non-neurogenic storage and voiding disorders with the chemical denervation caused by botulinum toxin type A- A pilot study. Neurourology & Urodynamics. 2001; 20: 410-2, Abstract.
- Zermann DH, Ishigooka M, Schubert J, Schmidt RA: Trigonum and bladder base injection of botulinum toxin A (BTX) in patients with severe urgency-frequency-syndrome refractory to conservative medical treatment and electrical stimulation. Neurourology & Urodynamics. 2001; 20: 412-3, Abstract.
- Chancellor MB, Smith CP: One surgeon’s experience in 50 patients with botulinum toxin injection into the bladder and urethra. J Urol. 2002; 167: 249.
- Carpenter FG: Motor responses of the urinary bladder and skeletal muscle in botulinum intoxicated rates. J Physiol. 1967; 188: 1.
- Bigalke H, Habermann E: Blockade by Tetanus and Botulinum A toxin of postganglionic cholinergic nerve endings in the myenteric plexus. Naunyn-Schmiedeberg’s Arch Pharmacol. 1980; 312: 255.
- Mackenzie I, Burnstock G, Dolly JO: The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience. 1982; 7: 997.
- Franks ME, Somogyi GT, Phelan MW, Fraser MO, Yokoyama T, Ghosh R, et al.: Botulinum toxin injection into the bladder wall decreases acetylcholine (ACh) and norepinephrine (NE) release: Potential treatment for the overactive bladder. J Urol. 2000; 163: 42, Abstract.
- Aoki KR: Pharmacology and Immunology of botulinum toxin serotypes. J Neurol. 2001; 248 (Suppl 1): 1/3-1/10.
- Smith CP, Fraser MO, Ghosh R, Lu S-H, de Groat WC, Chancellor MB, et al.: Botulinum toxin D is more potent than botulinum toxin A in inhibiting bladder contractions. APS 47th Annual Conference, 2001.
- Welch MJ, Purkiss JR, Foster KA: Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000; 38: 245-58.
- Purkiss JR, Welch MJ, Doward S, Foster KA, Quinn CP: A method for the measurement of [3H]-glutamate release from cultured dorsal root ganglion neurons. Biochem Soc Trans. 1998; 26: S108.
- Cui M, Aoki KR: Botulinum toxin type A (BTX-A) reduces inflammatory pain in the rat formalin model. Cephalagia. 2000; 20: 414.
- Smith CP, Fraser MO, Bartho L, de Groat WC, Chancellor MB, Somogyi GT: Botulinum toxin A inhibits afferent nerve evoked bladder strip contractions. J Urol. 2002; 167: 41.
- Smith
CP, Somogyi GT, Chancellor MB: Botulinum toxin: Poisoning the spastic
bladder and urethra. Rev Urol. 2002; 4: 61-8.
____________________
Received: May 28, 2002
Accepted: June 28, 2002
_______________________
Correspondence address:
Dr. Michael B. Chancellor
University of Pittsburgh School of Medicine
700 LS Kaufmann Building
3471 Fifth Avenue
Pittsburgh, Philadelphia, 15213, USA
Fax: + 1 412 692-4081
E-mail: chancellormb@msx.upmc.edu