ANALYSIS
OF RISK FACTORS OF INVOLVEMENT OF SEMINAL VESICLES IN PATIENTS WITH PROSTATE
CANCER UNDERGOING RADICAL PROSTATECTOMY
(
Download pdf )
MARCOS F. DALL’OGLIO, ALEXANDRE C. SANT’ANNA, ALBERTO A. ANTUNES, LUCIANO J. NESRALLAH, KATIA R. LEITE, MIGUEL SROUGI
Division
of Urology, Paulista School of Medicine, Federal University of São
Paulo, UNIFESP, and
Section of Pathology, Syrian Lebanese Hospital, São Paulo, SP,
Brazil
ABSTRACT
Objective:
To determine through preoperative serum PSA level, Gleason score on biopsy
and percentage of fragments affected by tumor on biopsy, the probability
of involvement of the seminal vesicles.
Materials and Methods: During the period
between March 1991 to December 2002, we selected 899 patients undergoing
radical prostatectomy for treatment of localized prostate adenocarcinoma.
The analyzed preoperative variables were PSA, percentage of positive fragments
and Gleason score on the biopsy. Pre-operative PSA was divided in scales
from 0 to 4.0 ng/mL, 4.1 to 10 ng/mL, 10.1 to 20 ng/mL and > 20 ng/mL,
Gleason score was categorized in scales from 2 to 6. 7 and 8 to 10, and
the percentage of affected fragments was divided in 0 to 25%, 25.1% to
50%, 50.1% to 75%, and 75.1% to 100%. All these variables were correlated
with the involvement of seminal vesicles in the surgical specimen.
Results: Of the 899 patients under study,
approximately 11% (95% CI, [9% - 13%]) had involvement of seminal vesicles.
On the multivariate analysis, when PSA was £ 4, the Gleason score
was 2 to 6, and less than 25% of fragments were involved on the biopsy,
only 3.6%, 7.6% and 6.2% of patients respectively, had involvement of
seminal vesicles. On the multivariate analysis, we observed that PSA,
Gleason score and the percentage of involved fragments were independent
prognostic factors for invasion of seminal vesicles.
Conclusion: The preoperative variables used
in the present study allow the identification of men with minimal risk
(lower than 5%) if involvement of seminal vesicles.
Key
words: prostatic neoplasms; neoplasm staging; prostate-specific
antigen; biopsy; seminal vesicle
Int Braz J Urol. 2004; 30: 472-8
INTRODUCTION
Radical
prostatectomy (RP) is the most effective approach for treating localized
prostate cancer (PCa) (1). However, almost 30% of patients will present
biochemical recurrence of the disease on the long term (2). The involvement
of seminal vesicles constitutes one of the main prognostic factors following
RP (2-4), since these patients present higher incidence of lymph nodal
metastases and rates of biochemical recurrence in up to 60% of cases within
5 years (3,4). Moreover, (6 to 26%) of patients undergoing RP have involvement
of the seminal vesicles (4,5), and for all these reasons, since its original
description in 1905 by Young (6), the classic technique of retropubic
RP involves the en-bloc removal of all prostate and seminal vesicles.
With the advent of the prostate specific
antigen (PSA), the majority of patients are diagnosed in early stages
of disease, and currently more than 60% are classified as T1c (7). Thus,
less frequently patients undergoing RP present involvement of seminal
vesicles, and their resection may be unnecessary in more than 90% of cases
(5).
Since the removal of the seminal vesicles
can be technically difficult in some cases, increasing the surgical time
and blood loss, and their removal can exert some influence on the erectile
function and recovery of urinary continence, in many patients (3,8,9),
the preoperative identification of cases with low risk of involvement
of seminal vesicles might select cases for surgery with preservation of
seminal vesicles, improving the quality of life of these patients following
the surgery.
Since digital rectal examination of the
prostate, imaging studies, and biopsy of the seminal vesicles fail to
detect the involvement of seminal vesicles in patients with localized
PCa (3), the preoperative prognostic factors more commonly used for predicting
tumor extension are PSA, Gleason score and clinical staging (7). Recently,
some authors proposed that in patients with PSA lower than to 10.0 ng/mL,
when the Gleason score was lower than 7 or less than 50% of fragments
were involved, there would be a risk of involvement of seminal vesicles
lower than 5%, and they could be preserved during RP (5).
In the present study, the objective was
to assess the probability of involvement of seminal vesicles using preoperative
PSA, Gleason score and the percentage of positive fragments on prostate
biopsy.
MATERIALS AND METHODS
During
the period from March 1991 to December 2002, we selected 960 patients
undergoing RP for management of localized PCa at Syrian Lebanese Hospital,
São Paulo, Brazil. The same surgeon performed all procedures and
the same pathologist performed the pathologic analyses of the specimen.
Data from the 960 selected patients, as
well as the number of fragments removed on biopsy, the number of fragments
with cancer, Gleason score, PSA and the pathological study of the surgical
specimen were retrieved from our database.
We excluded 54 patients who received neoadjuvant
treatment and 7 who were diagnosed through endoscopic resection of the
prostate or transvesical prostatectomy, leaving a total of 899 patients.
All patients underwent clinical staging, using the TNM classification
(AJCC, 1992), using diagnostic testes that included dosage of serum PSA,
digital rectal examination, transrectal prostate biopsy, abdominal and
pelvic computerized tomography, bone scintigraphy and chest radiography.
Preoperative variables analyzed were PSA,
the percentage of positive fragments and Gleason score on biopsy. Preoperative
PSA was categorized in scales from 0 to 4.0 ng/mL, 4.1 to 10 ng/mL, 10.1
to 20 ng/mL and > 20 ng/mL, and the Gleason score was divided in scales
from 2 to 6. 7 and 8 to 10. The percentage of positive fragments was defined
as the number of fragments with cancer divided by the total number of
fragments on biopsy, divided in ranges from 0 to 25%, 25.1% to 50%, 50.1%
to 75%, 75.1% to 100%. All these variables were related to the involvement
of the seminal vesicles in the surgical specimen.
Mean age was 62.8 ± 7.4 years (ranging
from 40 to 83 years). Mean PSA was 10.1 ± 7.7 ng/mL (ranging from
0.3 to 72 ng/mL). In relation to the clinical stage, 432 (48%) patients
were classified as T1c, 219 (24%) as T2a, 173 (19.3%) as T2b, 68 (7.6%)
as T2c and 7 (0.8%) as T3a. The mean percentage of affected fragments
was 41% ± 24% (ranging from 5% to 100%). Mean Gleason score on
biopsy was 5.8 ± 1.3, ranging from 2 to 9.
RP specimens (prostate, seminal vesicles
and obturator lymph nodes) were fixed, in average during 6 hours, in 10%
formalin and underwent a routine consisting of measurement and weighting
of the gland in a digital balance sensitive to 2 decimal places. Thin
transversal sections were made on the surgical margins relative to the
bladder neck and prostate apex. Considering the urethra for reference,
the remaining gland, after its margins had been stained with India ink,
were sequentially sliced at each 3 millimeters. Eight to 10 sections from
each lobe were included for the histological study. The seminal vesicles
were sectioned at the base, and were prepared for histological examination
after longitudinal sectioning. The involvement of seminal vesicles was
considered only when there was parenchymal invasion, without considering
adventitial invasion.
Statistical assessment was performed through
Pearson qui-square test and trend test for univariate analysis. For multivariate
analysis, an approach of logistic regression was used. A significance
level of 5% was adopted, thus, p values < 0.05 were considered statistically
significant.
RESULTS
Of
the 899 patients under study, approximately 11% (95% CI, 9% - 13%) had
involvement of seminal vesicles. In relation to preoperative PSA, we observed
that 9.3% (84) of the total of assessed patients presented levels lower
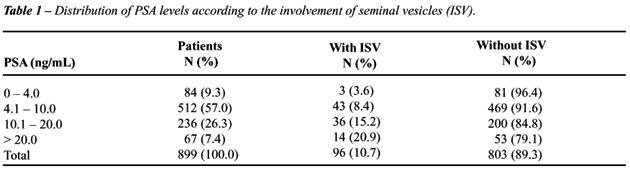
or equal to 4.0 ng/mL. Table-1 shows the distribution of patients according
to the involvement of seminal vesicles and preoperative PSA levels. The
likelihood of presenting involvement of seminal vesicles increases according
to the increase in preoperative serum PSA levels (p < 0.001).
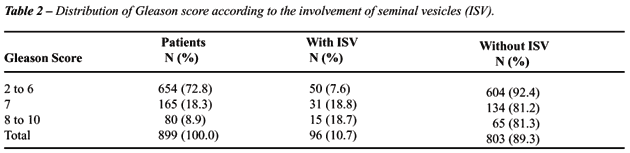
Gleason score on biopsy showed association
with the involvement of seminal vesicles as well (p < 0.001). Observing
the Table-2 we verified that there is no statistically significant difference
between the distribution of Gleason score 7 and 8 to 10 (p = 0.994), therefore
we constructed a new category for scores between 7 to 10. According to
the Table-3, approximately 8% of patients with Gleason score between 2
and 6 had involvement of seminal vesicles. On the other hand, among men
with Gleason score between 7 and 10, 46 (19%) out of 245 had involvement
of seminal vesicles.

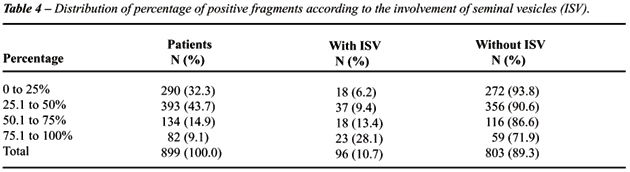
The qui-square test showed a significant
association between the percentage of affected fragments and involvement
of seminal vesicles (p < 0.001). The Table-4 shows that as the percentage
of affected fragments increase there is an increase in the proportion
of patients with involvement of seminal vesicles (p < 0.001).
PSA levels, Gleason score and the percentage
of positive fragments contributed significantly for predicting the involvement
of seminal vesicles in logistic regression (p < 0.001). All second-
and third-order interactions were tested and shown to be non significant
(p > 0.05). Thus, the final model included only the main effects, which
were shown to be independent prognostic factors for involvement of seminal
vesicles.
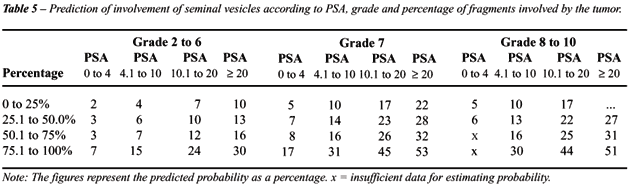
The figures presented in each cell of Table-5
represent the probability of involvement of seminal vesicles based on
logistic regression with PSA, Gleason score and percentage of affected
fragments. For example, a man with PSA lower than or equal to 4.0 ng/mL,
percentage of affected fragments up to 75% and Gleason score between 2
and 6 has a chance of less than 5% of presenting involvement of seminal
vesicles. The same risk is observed for men with PSA lower than or equal
to 10 ng/mL, percentage of affected fragments up to 25% and Gleason score
of 6. On the other hand, men with PSA higher than 10 ng/mL, more than
75% of affected fragments and Gleason score above 6 have a risk higher
than 40% of presenting involvement of seminal vesicles.
COMMENTS
In
the present study, the authors demonstrated that in earlier cases, the
involvement of seminal vesicles is uncommon, and this probability can
be determined through the studied variables. Thus, in univariate analysis,
when PSA is £ 4, Gleason score is between 2 to 6 and less than 25%
of biopsy fragments are involved, only 3.6%, 7.6% and 6.2% of patients
respectively, will have involvement of seminal vesicles.
Among advantages reported in the literature
concerning the preservation of seminal vesicles is the improvement in
urinary continence and postoperative sexual potency (8,9). Additionally,
some cases present technical difficulties during the resection, increasing
surgical time and blood loss (3).
Rates of post-RP urinary continence decreased
with the increasing anatomic knowledge on neurovascular bundles. Moreover,
better technical improvement, especially during dissection of the prostate
apex, has enabled better preservation of the extrinsic sphincteric muscles
(10). Despite these advances, urinary incontinence continues to impair
the quality of life of some patients, and can reach 60% during the first
6 months after surgery (10).
Accurate surgical measures that influence
post-RP urinary continence are not fully understood, and recent studies
suggest that trigone innervation and posterior urethral sensitivity play
an important role in continence during the immediate postoperative period
(8). These nerve branches can be damaged during dissection of the prostate,
posterior aspect of bladder and seminal vesicles, contributing for sphincteric
incompetence (8). Thus, the preservation of seminal vesicles would prevent
traction and damage to such structures, improving immediate postoperative
continence.
Jonh et al. (8) studied the urinary continence
in 20 patients undergoing RP with preservation of seminal vesicles and
compared their results to other 34 patients undergoing classic RP. They
observed that in the group with preservation of seminal vesicles, the
continence rates at 6 weeks and 6 months following surgery were 60% and
95% respectively, versus 18% and 82% during the same periods for the group
undergoing classic surgery.
The removal of seminal vesicles during RP
is also partially implied as a cause of erectile dysfunction in many patients
(3,8). Sexual function can be affected after RP despite the use of techniques
that preserve the neurovascular bundles, showing that other mechanisms
are involved in this process. The close relationship between the seminal
vesicles and the lateral prostate pedicles, with penile blood supply and
their own neurovascular bundles, suggest that the resection of the seminal
vesicles can actually contribute for postoperative erectile dysfunction
(5). Studies on sexual function with patients undergoing RP techniques
with preservation of the seminal vesicles show a clear advantage when
compared with patients undergoing classic RP. In a series of 191 patients
that were prospectively assessed, the authors observed that sexual health
in relation to the quality of life was significantly better in patients
with preservation of seminal vesicles when compared with patients undergoing
classic surgery (9).
In addition to these factors, the low frequency
of PCa invasion to the seminal vesicles often makes their removal unnecessary.
One study assessing the surgical specimens from 71 consecutive patients
undergoing RP, found 12 cases (17%) of involvement of seminal vesicles,
with 5 bilateral cases, but in none of theses cases the tumor had spread
to the distal 1 cm of seminal vesicles. The authors comment that in cases
of technical difficulty, even if a small fragment of seminal vesicles
is not removed, the patient’s prognosis probably will not change
(11).
Thus, it is clear that preoperative identification
of patients with involvement of seminal vesicles, in addition to being
an important factor for staging and prognosis, can help to select cases
for preservation of the seminal vesicles. In patients with localized PCa,
imaging studies have limited accuracy and the biopsies of seminal vesicles
have negative, positive predictive value and sensitivity of only 84 to
97%, 80% and 67% respectively (3). Thus it is necessary to identify risk
factors for involvement of seminal vesicles, with main preoperative prognostic
factors and serum PSA levels, Gleason score on biopsy and clinical staging
(7).
The percentage of positive fragments on
biopsy is associated with pathological characteristics, biochemical progression,
distant metastases and overall survival in patients undergoing RP. Recent
works show that this parameter must be used in preoperative models for
predicting the prognosis (12). Peller et al. (13), while studying the
usefulness of transrectal prostate biopsy for determining tumor extension
of PCa in 102 patients undergoing RP, observed that the number of positive
fragments was correlated with the involvement of seminal vesicles. Thus,
when up to 50% or fragments were affected, only 10% presented involvement,
versus 57% when more than 50% of fragments were affected (13). The present
study demonstrated that similarly, when no more than 50% of fragments
are affected, the risk of involvement of seminal vesicles is 10%, however
when more than 50% of fragments are affected, such risk is superior to
28%.
The presence of positive fragments removed
from the prostate base is also correlated with involvement of seminal
vesicles. In a study with 763 patients with clinical stage T1c to T3 undergoing
RP, 437 patients presented positive fragments at the base and 12.8% of
those had involvement of seminal vesicles, versus only 1.2% of 326 patients
without PCa at the base. On the multivariate analysis, serum PSA, primary
Gleason grade, and the percentage of PCa at the base were the only prognostic
factors implied in the involvement of seminal vesicles (3).
Zlotta et al. (5), in a retrospective study
with 1238 patients, observed that when PSA was < 10.0 ng/mL, only 5.2%
had involvement of seminal vesicles. On the multivariate analysis, the
percentage of affected fragments on biopsy and Gleason score were predictive
factors for involvement of seminal vesicles in this group of patients.
Thus, in patients with PSA < 10.0 ng/mL, when the Gleason score was
< 7 or less than 50% of the fragments were involved, there was a risk
lower than 5% of involvement of seminal vesicles. In the present series,
when the PSA was £ 10 ng/mL, we observed an 8.4% risk of involvement
of seminal vesicles on univariate analysis. On the multivariate analysis
we observed only 2 situations where the risk of involvement of seminal
vesicles was lower than 5%, that is, PSA up to 4 ng/mL with a maximum
of 75% of fragments affected by tumor and Gleason score lower than or
equal to 6, and PSA under 10 ng/mL with up to 25% of fragments affected
and Gleason score of 6 at a maximum. In no case with more than 75% of
affected fragments or Gleason score higher than 6, there was a risk lower
than 5% for involvement of seminal vesicles.
Since we still do not know the long-term
effects of preserving the seminal vesicles, in the future this study could
help to create study protocols that enable their preservation. The authors
still recommend en-bloc removal of the seminal vesicles during RP however,
in selected cases presenting the parameters mentioned above, non-removal
of the entire seminal vesicles probably will not impair patients’
prognosis due to the low risk for their involvement.
_________________________________________
Adriana Sañudo performed the statistical analysis
REFERENCES
- Cooperberg MR, Broering JM, Latini DM, Litwin MS, Wallace KL, Carroll PR: Patterns of practice in the United States: insights from PCaSURE on prostate cancer management. Curr Urol Rep. 2004; 5: 166-72.
- Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT: Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002; 7: 528-34.
- Koh H, Kattan MW, Scardino PT, Suyama K, Maru N, Slawin K, et al.: A nomogram to predict seminal vesicle invasion by the extent and location of cancer in systematic biopsy results. J Urol. 2003; 170: 1203-8.
- Sofer M, Savoie M, Kim SS, Civantos F, Soloway MS: Biochemical and pathological predictors of the recurrence of prostatic adenocarcinoma with seminal vesicle invasion. J Urol. 2003; 169: 153-6.
- Zlotta AR, Roumeguere T, Ravery V, Hoffmann P, Montorsi F, Turkeri L, et al.: Is seminal vesicle ablation mandatory for all patients undergoing radical prostatectomy? A multivariate analysis on 1283 patients. Eur Urol. 2004; 46: 42-9.
- Young HH: The early diagnosis and radical cure of carcinoma of the prostate. Being a study of 40 cases and presentation of a radical operation which was carried out in four cases. 1905. J Urol. 2002; 167: 939-46; discussion 947.
- Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD: Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001; 58: 843-8.
- John H, Hauri D: Seminal vesicle-sparing radical prostatectomy: a novel concept to restore early urinary continence. Urology. 2000; 55: 820-4.
- Sanda MG, Dunn R, Wei J: Seminal vesicle sparing technique is associated with improved sexual HRQOL outcome after radical prostatectomy. J Urol. 2002; 167: 151, A606.
- Steiner MS, Morton RA, Walsh PC: Impact of anatomical radical prostatectomy on urinary continence. J Urol. 1991; 145: 512-4; discussion 514-5.
- Korman HJ, Watson RB, Civantos F, Block NL, Soloway MS: Radical prostatectomy: is complete resection of the seminal vesicles really necessary? J Urol. 1996; 156: 1081-3.
- Lotan Y, Shariat SF, Khoddami SM, Saboorian H, Koeneman KS, Cadeddu JA, et al.: The percent of biopsy cores positive for cancer is a predictor of advanced pathological stage and poor clinical outcomes in patients treated with radical prostatectomy. J Urol. 2004; 171: 2209-14.
- Peller PA, Young DC, Marmaduke DP, Marsh WL, Badalament RA: Sextant prostate biopsies. A histopathologic correlation with radical prostatectomy specimens. Cancer. 1995; 75: 530-8.
__________________________
Received:
September 13, 2004
Accepted after revision: October 27, 2004
_______________________
Correspondence address:
Dr. Marcos F. Dall’Oglio
Rua Barata Ribeiro, 398 / 5o.
São Paulo, SP, 01308-000, Brazil
Fax: + 55 11 3159-3618
E-mail: marcosdallogliouro@terra.com.br