DEVELOPMENT
OF A COMPUTER ASSISTED GANTRY SYSTEM FOR GAINING RAPID AND ACCURATE CALYCEAL
ACCESS DURING PERCUTANEOUS NEPHROLITHOTOMY
(
Download pdf )
A. D. ZARRABI, J. P. CONRADIE, C. F. HEYNS, C. SCHEFFER, K. SCHREVE
Department of Urology (ADZ, CFH), University of Stellenbosch and Tygerberg Hospital, Tygerberg, South Africa and Department of Mechanical and Mechatronic Engineering (JPC, CS, KS), University of Stellenbosch, Stellenbosch, South Africa
Basic and Translational Urology
Vol. 36 (6):
738-748, November - December, 2010
doi: 10.1590/S1677-55382010000600013
ABSTRACT
Purpose:
To design a simple, cost-effective system for gaining rapid and accurate
calyceal access during percutaneous nephrolithotomy (PCNL).
Materials
and Methods: The design consists of a low-cost, light-weight, portable
mechanical gantry with a needle guiding device. Using C-arm fluoroscopy,
two images of the contrast-filled renal collecting system are obtained:
at 0-degrees (perpendicular to the kidney) and 20-degrees. These images
are relayed to a laptop computer containing the software and graphic user
interface for selecting the targeted calyx. The software provides numerical
settings for the 3 axes of the gantry, which are used to position the
needle guiding device. The needle is advanced through the guide to the
depth calculated by the software, thus puncturing the targeted calyx.
Testing of the system was performed on 2 target types: 1) radiolucent
plastic tubes the approximate size of a renal calyx (5 or 10 mm in diameter,
30 mm in length); and 2) foam-occluded, contrast-filled porcine kidneys.
Results:
Tests using target type 1 with 10 mm diameter (n = 14) and 5 mm diameter
(n = 7) tubes resulted in a 100% targeting success rate, with a mean procedure
duration of 10 minutes. Tests using target type 2 (n = 2) were both successful,
with accurate puncturing of the selected renal calyx, and a mean procedure
duration of 15 minutes.
Conclusions:
The mechanical gantry system described in this paper is low-cost, portable,
light-weight, and simple to set up and operate. C-arm fluoroscopy is limited
to two images, thus reducing radiation exposure significantly. Testing
of the system showed an extremely high degree of accuracy in gaining precise
access to a targeted renal calyx.
Key
words: nephrolithotomy; percutaneous; access; computers; endourology;
urolithiasis
Int Braz J Urol. 2010; 36: 738-48
INTRODUCTION
Since
the first description of percutaneous nephrolithotomy (PCNL) by Fernström
and Johansson more than 30 years ago, major technological advances have
improved the efficacy and safety of this procedure, confirming its superiority
compared to open surgery for renal calculi (1).
Obtaining precise access to a predetermined renal calyx is the most critical
part of PCNL (2). Currently available techniques for obtaining percutaneous
(PC) access to the renal collecting system include the following:
Two-stage
procedure: Pre-operative access is first obtained by an interventional
radiologist using ultrasound guidance, after which the urologist dilates
the tract and performs the PCNL. A recent report indicates that the minority
of urologists (11%) gain their own access for PCNL (3).
Retrograde percutaneous access: This involves retrograde placement of
a ureteric catheter, followed by passage of a sharp wire through the catheter
and via the selected calyx to the skin (4). Despite the feasibility of
this method, it offers no advantage over antegrade percutaneous access
and is not commonly utilized.
Fluoroscopic X-ray guided techniques: The two techniques best described
are “eye of the needle” and “triangulation”. Both
consist of several steps where C-arm fluoroscopy is rotated into different
positions relative to the needle and the target (contrast-filled calyx)
(2). The needle is advanced until the calyx is punctured in a controlled
and predictable fashion. These are the access techniques most commonly
used by urologists. However, gaining access to a pre-identified calyx
is usually the most difficult part of PCNL. It often requires multiple
needle punctures and prolonged radiological screening, and sub-optimal
access leads to increased operative times and decreased stone-free rates.
Robotic
assisted access: The first robotic system to access the renal collecting
system for PCNL was described by Potamianos et al. in 1995 and consisted
of a manually positioned robotic arm mounted on the operating table, guided
by C-arm fluoroscopy (5,6). Cadeddu et al. designed a fully automated
robot that managed all aspects of PC access: planning the needle trajectory,
needle positioning and advancement and real-time needle tracking using
biplanar fluoroscopy (7). Stoianovici et al. developed a metal arm with
6 degrees of freedom movement that was manipulated mechanically by the
urologist. It was attached to the side rail of the operating table, and
incorporated a radiolucent needle grasping disc at the distal end of the
arm (8). The PAKY (percutaneous access to the kidney) system and later
PAKY-RCM (remote center of motion) system were improvements on the original
design: the passive robotic arm was improved by adding an electronic needle
insertion device and the ability to position the needle by remote control
(9,10).
In
all the abovementioned designs, except the fully automated robot, the
“robotic arms” mainly serve to stabilize and in some cases
advance the needle, and reduce radiation exposure. However, the urologist
still needs to calculate and plan the needle trajectory to the desired
renal calyx.
Therefore, the challenge is to develop a system that is cost-effective
and simple to use, yet faster and more accurate than is possible for the
average general urologist. Prototypes of a fully automated robot managing
all aspects of access for PCNL have been built, but their size and complexity
have prevented application in routine clinical practice (7).
The
aim of this study was to design a simple and cost-effective system for
use by urologists to gain rapid and accurate percutaneous access for performing
PCNL. Although an automated system provides rapid needle alignment and
insertion, active components increase system costs. Furthermore, bi-planar
fluoroscopy is not as commonly used in operating rooms as mobile C-arm
systems. This paper describes the development of a computer directed gantry
system using C-arm fluoroscopy to gain rapid and accurate renal access
during PCNL.
MATERIALS AND METHODS
This
access system is based on the principle of triangulation - the process
of localizing a point in 3 dimensions by using 2 intersecting lines. In
the setting of PCNL, the fluoroscopic images of the contrast-filled renal
calyceal system obtained intra-operatively are used. One image of the
collecting system is obtained with the C-arm in the 0-degrees position
(directly perpendicular to the kidney) and the other with the C-arm tilted
20-degrees. The images are taken at the same time in the respiratory cycle
during controlled mechanical ventilation.
These
images are relayed to a laptop computer containing the software and graphic
user interface. The ideal setup would be a system where the C-arm has
an output connected to the laptop so that the images can be transferred
there directly. Due to limitations of our C-arm unit, this was unfortunately
not possible and images had to be saved on a “flash drive”
and manually imported to the graphic user interface on the laptop. On
these images, the urologist marks the specific area of the specific calyx
targeted for puncturing by clicking with the computer mouse on the fluoroscopic
images. The software calculates the needle trajectory and subsequently
provides the numerical settings for the 3 axes of the gantry, which is
attached to the operating table. The needle positioning device on the
gantry is set into position by the urologist according to the values provided
by the software. The needle is now placed through the needle guide and
inserted to the depth calculated by the software, which should result
in puncturing of the targeted calyx.
Hardware
Needle Positioning Device
The central feature of this design is a needle alignment device and portable, light-weight mechanical gantry that is attached to the edge of the operating table (Figure-1). The gantry contains no motorized parts or electronic components. The system is manually adjusted by the urologist and consists of 3 orthogonal axes and a gyro-like end-effector resulting in a 6 degrees of freedom system.
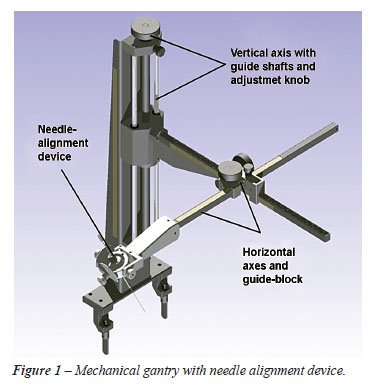
The
vertical axis of the system carries the combined weight of the other axes
and end-effector, and is attached to the existing rails on the theatre
table. High precision guide shafts with closed linear bushings ensure
accuracy. An adjustment resolution of 2 mm per knob rotation is achieved.
A vertical translation range of 400 mm is possible, which is more than
adequate to accommodate patients with varying body habitus.
A
rack and pinion option provides flexibility to the design and simplifies
placement of fixing knobs for the horizontal axes. Bending and torsion
forces are addressed by a web and a counter balance, which prevents twisting
of the rack. A guide-block, housing the pinions, adjustment- and fixing
knobs, ensures high precision linear movement of the 2 rack and pinion
configurations. The horizontal axes cover a 450 mm x 450 mm area at the
height of the vertical axis.
The
gyro mechanism or needle-alignment mechanism (end-effector) orientates
the needle around a fixed point (Figure-2). Aligning a needle with a specified
vector requires 2 rotational degrees of freedom. As the needle-alignment
mechanism will be positioned over the kidney, a radiolucent acrylic (Perspex)
was used in the manufacturing of the mechanism. It consists of 3 main
components for the 2 axes of rotation: a fixed base, an outside ring for
rotation around the y-axis and an inside ring for rotation around the
x-axis. The rings are locked in position by friction locks. Engraved dials
display the angle of needle rotation, which ranges ± 40-degrees
around both axes, resulting in the needle rotation range shown in Figure-3.
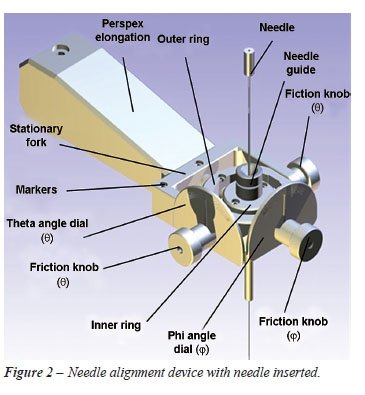

Two
groups of 2 mm diameter stainless steel navigation markers, required for
coordinate identification and needle manipulation, are located on the
radiolucent needle-alignment mechanism. These are called the gantry and
needle marker groups. The needle is gripped by a friction mechanism, which
allows the urologist to adjust the friction by which the needle is held
during insertion.
Software
Software
was designed using Python® (which can be obtained via free internet
download) and was run on a standard laptop computer.
User
interface - The control center of the positioning system is the urologist-operated
user interface, aiding in the calibration and the targeting procedure.
Calibration
screen - The user is required to select and import the calibration images.
The calibration algorithm is performed automatically and takes approximately
15 seconds to complete.
Point
selection screen - All points on the specified calyx required for targeting
are identified and selected by the urologist - this provides the calyx
markers.
Targeting
screen - This shows the final translation and rotation (i.e. numerical
settings of the 3 axes of the gantry) required for targeting.
•
Insertion routines - Two insertion routines can be utilized with the information
gained (Figure-4).
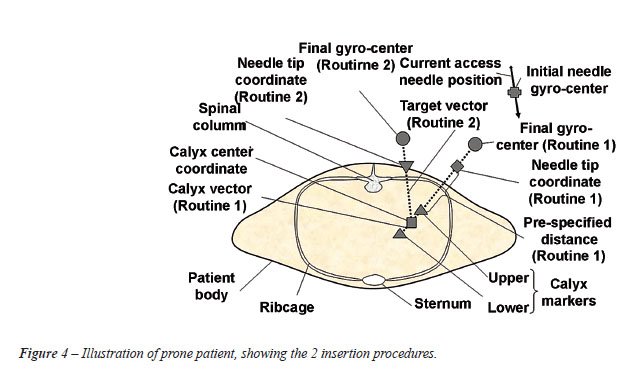
•
Insertion routine 1 uses the defined calyx vector and needle tip coordinate
and attempts to orientate the needle in this vector at a pre-specified
distance from the calyx tip (Figure-4). Routine 1 resembles the “triangulation”
technique.
•
Insertion routine 2 uses the needle tip coordinate and calyx center coordinate
to provide a new vector to which the needle is adjusted (Figure-4). Routine
2 resembles the “eye of the needle” technique.
As
the targeted gyro-center coordinates for the two respective insertion
routines are known, the required translation from the initial gyro-center
coordinate to any of the targeted markers can be computed. The operator
can select either one of the two insertion routines. The required translation
in the gantry x-, y-, z directions for the respective routines is calculated
as the difference between the transformed coordinates of the target and
initial gyro mechanism center coordinates:
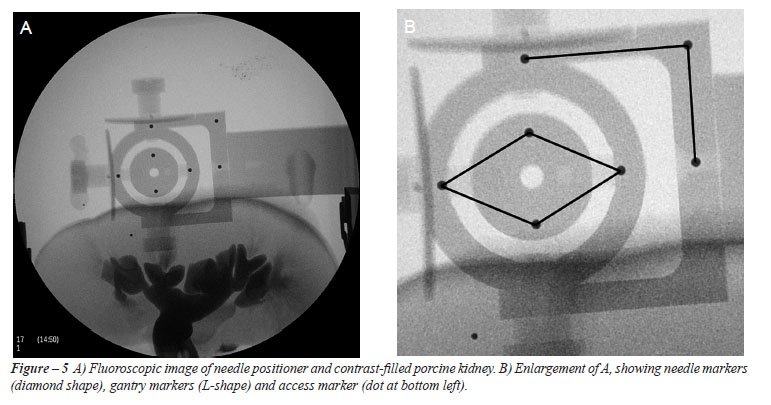
Marker
Selection - Three groups of navigation markers require selection (Figure-5).
They are called the gantry, needle and access marker groups, respectively.
The needle markers are visible as a diamond shaped configuration. The
gantry markers on their outside are in the shape of a flipped capital
letter “L”. The access marker shows as a small individual
artifact which is easily recognized (Figure-5B). The needle markers serve
a dual function: they define the needle orientation and the center of
rotation of the gyro mechanism. The gantry markers define the movement
directions of the x, y and z translations of the needle positioning system.
The access marker defines the needle access point.
Determining
point correspondences of the kidney calyx in the stereo image pair is
problematic, as definite corresponding structures or points are not easily
discerned. A four or two-point selection method can be implemented (Figure-6).
In the four-point method, 2 points are selected at either end of one edge
of the calyx, and another 2 on the opposite edge of the calyx. The calyx
vector is determined by triangulation and subtraction of the coordinates
halfway between the 2 selected calyx-end coordinates (Figure-6). With
the two-point method, a point is selected centrally at the one end of
the calyx and another on the opposite end in the estimated center of the
calyx.
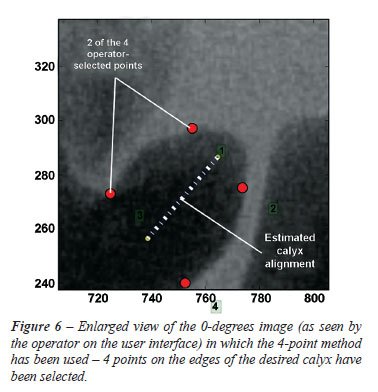
It
was found that the four-point method reduced the point selection error
in cases where calyx image edges were unclear (due to limitations of the
fluoroscopy system). In cases where a distinct edge could be identified,
the two-point method was adequate.
Experimental Setup for Testing
Laboratory
Testing
The
operating room environment was simulated using digital cameras in a configuration
resembling that of the C-arm fluoroscopy system (Figure-7). This allowed
thorough testing of the system in a controlled environment to determine
mechanical design accuracy and repeatability. This setup also allowed
a basis for algorithm testing during system development stages.

Operating
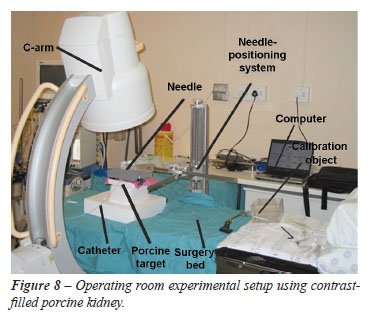
Room Testing
Testing
of the system using C-arm fluoroscopic imaging was performed on 2 target
types in an operating room (Figure-8).
Target
type 1 consisted of radiolucent acrylic plastic tubes, 5 and 10 mm in
diameter (approximate calyceal diameter) and 30 mm in length, with two
1 mm diameter stainless steel spheres attached on opposite sides of each
tube.
Target
type 2 consisted of a foam-occluded, contrast-filled porcine kidney -
resembling a model described by De Sa Earp for teaching PCNL access (11).
An occlusion balloon catheter was inserted into the ureter and blue-colored
radiological contrast medium introduced into the collecting system under
gravitational force.
Apart
from the imaging system and target differences, the same steps used during
the laboratory setup were followed. The operating room experimental setup
is shown in Figure-8.

RESULTS
The cost
of manufacturing the gantry and needle positioning mechanism was approximately
US$ 1,500. All other equipment (operating table, C-arm fluoroscopy, access
needle) were standard as for routine PCNL.
Fourteen tests were performed in the operating room using the 10 mm diameter
target type-1. Targeting done within 50 mm above and 50 mm below the calibrated
volume resulted in a 100% targeting success rate. Due to the large distortion
in the X-ray images, reconstruction of points too far outside the calibrated
volume resulted in large targeting errors.
Seven tests completed with the 5 mm diameter target type-1 resulted in
the same success rate. The targeting procedure took approximately 10 minutes
to complete for each of the respective type-1 targets.
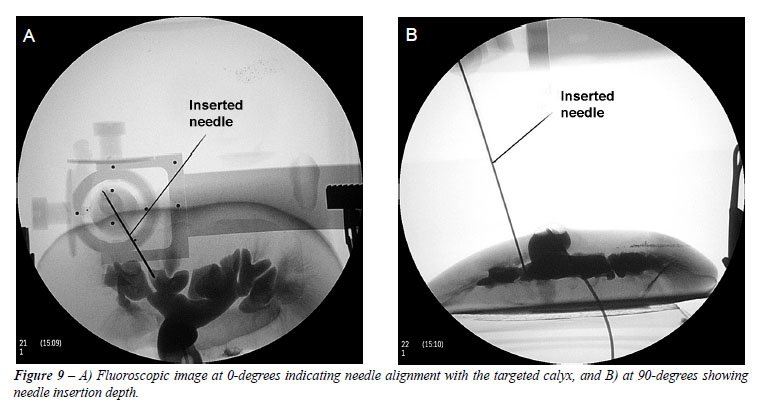
Two target type-2 procedures were performed. Successful needle insertion
was achieved in both cases and validated by aspiration of blue contrast
medium through the needle as well as fluoroscopic screening (Figure-9).
The targeting procedure for the type-2 targets took approximately 15 minutes
to complete, excluding the calibration process.
COMMENTS
PCNL
is the most difficult stone surgery technique for the trainee urologist
to learn (12). PC access performed by a radiologist is a practical option,
often employed. However, PC access related complications are fewer and
stone-free rates are higher with urologist-acquired access (3,13).
Training
on animals is difficult to organize, expensive and not sufficiently close
to human reality. Häcker et al. described teaching ultrasound- or
fluoroscopy guided PC renal access with a porcine kidney “hidden”
in a chicken carcass (14). Various virtual reality and inanimate simulators
for endourological training have been developed, but none specifically
for PC renal access (15).
Heyns and Van Gelderen (1990) were the first to propose computed tomography
imaging of the pelviocalyceal system with 3-dimensional reconstruction
as an aid in selecting the appropriate calyx for PCNL (16). Mozer et al.
superimposed ultrasound images onto fluoroscopic images to help plan PC
renal puncture (17). “Pelviocalyceal biomodeling” (18) and
“rapid prototyping” (19) have been described to create a replica
of individual patients’ stone-containing renal collecting systems,
on which PCNL and PC access can be planned and practiced prior to the
actual surgery.
The
robots and robotic arms discussed earlier are certainly accurate in obtaining
access. Unfortunately, they are still too large and/or expensive. Furthermore,
the majority of these systems still rely on the urologist to calculate
the trajectory of the needle to the desired calyx. Robots for PCNL have
been used in experimental settings only. Obtaining remote PC renal access
using an automated telesurgical robotic system has been successfully performed
(20). With this system, an experienced endourologist is only a phone call
away. This holds promise for the future.
CONCLUSIONS
The
mechanical gantry system described in this paper is low-cost, portable,
light-weight, sturdy and resilient. C-arm fluoroscopy is limited to two
images, thus reducing radiation exposure significantly. As there are no
electronic switches or dials involved in positioning the gantry and needle
alignment mechanism, it is simple to set up and operate. Testing of the
system, both in the laboratory and in the operating room with the use
of contrast filled porcine kidneys, showed an extremely high degree of
accuracy in gaining precise access to a targeted renal calyx. Further
study is required to validate its use during PCNL in humans.
Further
study is also required to improve some engineering and design features.
As C-arm imaging systems were not designed to produce stereoscopic images
for point triangulation, errors due to movement during the acquisition
of targeting images are expected. A method to accurately monitor and control
C-arm positioning is necessary. Another problem in image-guided systems
requiring calibration prior to targeting is the fact that only a specified
volume is calibrated. Point reconstruction outside this volume introduces
large errors. Factors such as needle deflection due to tissue resistance
and obstruction by adjacent structures were not addressed at this stage
of development.
ACKNOWLEDGEMENTS
Research
funding for this project was provided by the Urological Association of
South Africa.
The
authors would like to thank Malcolm April, Elsabé du Toit and Dr
Willem Groenewald from the Department of Medical Imaging and Clinical
Oncology at Tygerberg Hospital for their assistance and the use of the
BV Pulsera fluoroscopy system.
The
University of Stellenbosch, on behalf of the authors, is the intellectual
property owner of the design, manufacture and patent rights of the equipment
described in this paper.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Fernström I, Johansson B: Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol. 1976; 10: 257-9.
- Miller NL, Matlaga BR, Lingeman JE: Techniques for fluoroscopic percutaneous renal access. J Urol. 2007; 178: 15-23.
- Watterson JD, Soon S, Jana K: Access related complications during percutaneous nephrolithotomy: urology versus radiology at a single academic institution. J Urol. 2006; 176: 142-5.
- Lawson RK, Murphy JB, Taylor AJ, Jacobs SC: Retrograde method for percutaneous access to kidney. Urology. 1983; 22: 580-2.
- Potamianos P, Davies BL, Hibberd RD: Intra-operative imaging guidance for keyhole surgery: methodology and calibration. Proceedings of the First International Symposium on Medical Robotics and Computer Assisted Surgery, Pittsburgh, Pennsylvania. 1994; pp. 98-104.
- Potamianos P, Davies BL, Hibberd RD: Intra-operative registration for percutaneous surgery. Proceedings of the Second International Symposium on Medical Robotics and Computer Assisted Surgery, Baltimore, Maryland. 1995; pp. 156-64.
- Cadeddu JA, Bzostek A, Schreiner S, Barnes AC, Roberts WW, Anderson JH, et al.: A robotic system for percutaneous renal access. J Urol. 1997; 158: 1589-93.
- Stoianovici D, Cadeddu JA, Demaree RD, Basile SA, Taylor RH, Whitcomb LL at al.: An efficient needle injection technique and radiological guidance method for percutaneous procedures. Proc 1st Joint Conf CRVMed II & MRCAS III, Grenoble, France. 1997; pp. 295-8.
- Cadeddu JA, Stoianovici D, Chen RN, Moore RG, Kavoussi LR: Stereotactic mechanical percutaneous renal access. J Endourol. 1998; 12: 121-5.
- Su LM, Stoianovici D, Jarrett TW, Patriciu A, Roberts WW, Cadeddu JA, et al.: Robotic percutaneous access to the kidney: comparison with standard manual access. J Endourol. 2002; 16: 471-5.
- Earp PP: Percutaneous renal surgery--new model for learning and training. Int Braz J Urol. 2003; 29: 151-4.
- Tanriverdi O, Boylu U, Kendirci M, Kadihasanoglu M, Horasanli K, Miroglu C: The learning curve in the training of percutaneous nephrolithotomy. Eur Urol. 2007; 52: 206-11.
- Osman M, Wendt-Nordahl G, Heger K, Michel MS, Alken P, Knoll T: Percutaneous nephrolithotomy with ultrasonography-guided renal access: experience from over 300 cases. BJU Int. 2005; 96: 875-8.
- Häcker A, Wendt-Nordahl G, Honeck P, Michel MS, Alken P, Knoll T: A biological model to teach percutaneous nephrolithotomy technique with ultrasound- and fluoroscopy-guided access. J Endourol. 2007; 21: 545-50.
- Laguna MP, Hatzinger M, Rassweiler J: Simulators and endourological training. Curr Opin Urol. 2002; 12: 209-15.
- Heyns CF, van Gelderen WF: 3-dimensional imaging of the pelviocaliceal system by computerized tomographic reconstruction. J Urol. 1990; 144: 1335-8.
- Mozer P, Conort P, Leroy A, Baumann M, Payan Y, Troccaz J, et al.: Aid to percutaneous renal access by virtual projection of the ultrasound puncture tract onto fluoroscopic images. J Endourol. 2007; 21: 460-5.
- Radecka E, Brehmer M, Holmgren K, Palm G, Magnusson P, Magnusson A: Pelvicaliceal biomodeling as an aid to achieving optimal access in percutaneous nephrolithotripsy. J Endourol. 2006; 20: 92-101.
- Bruyère F, Leroux C, Brunereau L, Lermusiaux P: Rapid prototyping model for percutaneous nephrolithotomy training. J Endourol. 2008; 22: 91-6.
- Bauer J, Lee BR, Stoianovici D, Bishoff JT, Micali S, Micali F, et al.: Remote percutaneous renal access using a new automated telesurgical robotic system. Telemed J E Health. 2001; 7: 341-6.
____________________
Accepted
after revision:
May 7, 2010
_______________________
Correspondence
address:
Dr. A. D. Zarrabi
Department of Urology
University of Stellenbosch and Tygerberg Hospital
PO Box 19063, Tygerberg, 7505, South Africa
Fax: + 27 21 933-8010
E-mail: adzarrabi@gmail.com
EDITORIAL COMMENT
This article proposes
an interesting method for gaining the calyx in a percutaneous surgery
and offers some important aspects like a welcoming less exposure to irradiation,
and requires less expertise of the surgeon for accessing the urinary tract.
Since of beginning of percutaneous nephrolithotripsy it is well known
that the puncture of the urinary system is best done by the urologist.
He chooses the calyx that could offer the best access to the determined
surgery. When an other access becomes necessary in a more complex surgery,
the surgeon without any other assistance can perform the procedure.
However, in several services the urinary tract approach continues to be
done by invasive radiologists. For novice urologists and for those who
are radiologist dependent, the equipment and the software may be useful
since it could be really cost-effective, commercially available and clinically
tested. It is an additional way to give autonomy to urologists in percutaneous
surgery.
Dr. Anuar
I. Mitre
Division of Urology
University of Sao Paulo, USP
Sao Paulo, SP, Brazil
E-mail: anuar@mitre.com.br
EDITORIAL COMMENT
Undoubtedly, percutaneous
surgery for kidney stones is the most demanding procedure for a young
endourologist to learn. As a matter of fact, it is also cumbersome for
the expert endourologist to pass onto the apprentice the techniques of
the procedure in a stepwise and clear manner. Having said that, any effort
to unfold the tricks of this treatment modality is worth trying.
Simulators, animal models, “ex vivo” and “in vivo”
training models are being built and tested worldwide, but to date there
has not been a single one that has proven capable of reproducing the “real
life” challenges of the percutaneous nephrolithotomy. The present
study creates a fixed computerized geometrical concept of the procedure,
where landmarks are delimitated and loaded onto a computer that generates
angles and aligns the tip of the needle towards the target (chosen calyx).
The idea is very interesting and minimizes the mistakes of the human made
puncture, but it needs further testing in “in vivo” animal
models.
However, understanding the mechanism on which this study was based is
the first and most important step for performing a safe calyx puncture.
Basically, the triangulation technique has been reproduced by the authors,
and once one (reader) understands this concept, the puncture becomes less
enigmatical.
Dr. Renato
Nardi Pedro &
Dr. Nelson Rodrigues Netto Junior
Division of Urology, UNICAMP
Sao Paulo, SP, Brazil
E-mail: rnpedro@unicamp.br
EDITORIAL COMMENT
Nephrolithiasis
is a worldwide problem that accounts for significant morbidity and expense.
Indications for an active treatment of renal calculi go mainly according
to the stone size as well as the clinical symptoms. The goal of Nephrolithiasis
treatment is the complete removal of all stones from the pelviocalyceal
system with the lowest possible morbidity. Extracorporeal shockwave lithotripsy,
percutaneous nephrolitholapaxy and flexible ureterorenoscopy are the main
options for treatment.
Percutaneous nephrolithotomy has undergone an evolution in technique and
in equipment since its introduction in the late 1970s. This evolution
continues today and is evidenced by the numerous publications about the
technique.
Although ureteroscopy and shock wave lithotripsy predominate in the treatment
of urolithiasis, percutaneous nephrolithotomy continues to be an important
part of the urologist’s armamentarium. Percutaneous nephrolithotomy
is a minimally invasive surgery that causes minimal renal injury and maximizes
stone clearance, especially in patients with complex stone disease.
The authors in this paper design a simple, cost-effective system for gaining
rapid and accurate calyceal access during percutaneous nephrolithotomy.
They showed that with the system described, we can have a new project
with low-cost ($1500), portable, light-weight, that can help any other
urologist around the world.
It is very important that the C-arm fluoroscopy is limited to two images,
thus reducing the risk of all Urologists who live with radiation exposure.
As the authors concluded, “Further study is required to validate
its use during PCNL in the human” and improve the system. We will
be expecting the human results.
Dr. Mauricio
Rubinstein
Section of Urology
Federal University of Rio de Janeiro State
Rio de Janeiro, RJ, Brazil
E-mail: mrubins74@hotmail.com
EDITORIAL COMMENT
The authors are to be congratulated on their innovative work aimed at developing a reliable and simple technology to aid with accurate calyceal access during PCNL. Many urologists in the United States rely on radiologist to gain initial kidney access at the time of surgery. I hope that continued development of this technology or something similar that is inexpensive may help urologists gain their own precise access. Any excitement for this innovation must be tempered, however, by the lack of in vivo testing where muscle and fascial deformation as well as kidney movement may diminish calyceal puncture success. We await further animal and clinical reliability evaluations.
Dr.
Jeffrey Anthony Cadeddu
Department of Urology
UT Southwestern Medical Center
Dallas, Texas, USA
E-mail: jeffrey.cadeddu@utsouthwestern.edu