BLADDER
AUGMENTATION IN RABBITS WITH ANIONIC COLLAGEN MEMBRANE, WITH OR WITHOUT
UROTELIAL PRESERVATION. CISTOMETRIC AND HYSTOLOGIC EVALUATION
(
Download pdf )
FÁBIO G. O. LEPPER, TAMARA M. RAMOS, JOSÉ C. S. TRINDADE FILHO, FABIANA R. VALE, CARLOS R. PADOVANI, GILBERTO GOISSIS
Department of Urology, Botucatu School of Medicine, State University of São Paulo (UNESP), Botucatu, São Paulo, Brazil
ABSTRACT
Introduction:
The use of bowel segments to perform bladder augmentation is associated
with several metabolic and surgical complications. A great variety of
synthetic materials, biodegradable or not, have been tested. Collagen-based
biomaterials have shown effectiveness for the regeneration and obtainment
of a functional bladder.
Objective: Assess the functional and histological
response of the rabbit bladder to anionic collagen membrane (ACM), either
when it is anastomosed to the bladder or it is placed onto bladder after
vesicomyectomy.
Materials and Methods: In 15 male rabbit
a partial cystectomy was performed. After 4 weeks they were divided in
3 groups. Group 1 (G1) – bladder augmentation with ACM. Group 2 (G2)
ACM is placed onto bladder after vesicomyectomy. Group 3 (G3) control
group. Maximal bladder capacity (MBC) and weight were assessed with 4
(M1), 8 (M2) and 12 (M3) weeks after partial cystectomy. In M3 was performed
the sacrifice and extraction of the bladder and kidneys for anatomopathologic
study.
Results: There were neither bladder stones,
nor implant extrusion in M3. There was a significant increase in MBC in
G1 and G2 (p<0.05), but no statistical differences in G3 (p=0.35).
There is no significant difference comparing G1 and G2. In M3, both groups
have shown a bigger MBC than G3 (p<0.05). The microscopic assessment
showed an inflammatory reaction in the bladder augmented, with urothelium
preserved.
Conclusions: The ACM was effective for the
increase of MBC. The bladders with preservation of the urothelium have
shown an extensive inflammatory process.
Key words:
bladder; transplant; collagen; rabbit model; urodynamics
Int Braz J Urol. 2002; 28: 464-70
INTRODUCTION
Several
congenital or acquired diseases promote anatomic or functional alterations,
turning difficult or precluding the reservoir function of the bladder
(1). When conservative therapies are not effective, bladder augmentation
or urinary diversion are recommended as alternative therapeutics (2).
Today, enterocystoplasty is the method most utilized to bladder augmentation
using ileum, colon or stomach segments (3). In spite of the success obtained
by these techniques, aggression to gastrointestinal tract may lead to
nutritional and electrolyte alterations, peritoneal adherences, abscesses,
enteric fistulae, excessive mucus production, bacterial colonization and
cancer (4,5).
In order to minimize these complications,
a variety of biologic or synthetic materials have been used in experimental
assays, as alternative methods for bladder augmentation (6). Most of them
presented complications, as lithiasis formation, infections, graft rejection
or extrusion, and no adequate bladder reconstruction (7). Collagen-based
biodegradable materials showed effectiveness to regenerate and normalize
bladder’s functional capacity (8,9).
With this aim, we have investigated anionic
collagen membrane to bladder augmentation, evaluating histologic and urodynamic
aspects. We have also tried to evaluate if maintaining the urothelium
intact would help on bladder regeneration.
MATERIALS AND METHODS
Acellular
anionic collagen membrane (Figure-1) was obtained through processing bovine
serosa, by a technique previously described, and maintaining it in sterile
recipients, from 4 to 10oC (10). For the performance of surgical procedures,
animals were anesthetized with 3,5% pentobarbital sodium, 1 mL/kg by parenteral
route.
Fifteen male rabbits were utilized, whose
weight varied from 3.1 to 4.2 (mean 3.6±0.3kg), initially submitted
to subtotal cystectomy (incision 1.0 cm above both ureteral meatus), with
bladder dome excision. Bladder edges and the abdominal wall were sutured
with running 4-0 chromic suture in separated layers. After 04 weeks, at
moment 0 (M0), the animals were distributed in three experimental groups
with five rabbits each. In Group 1 (G1) we performed detrusorectomy according
to the technique described (11) and, over the mucosa, we have sutured
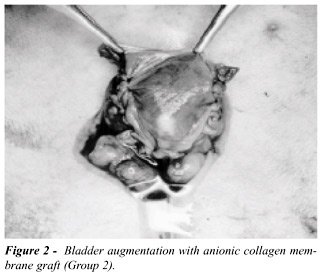
the collagen membrane, with approximately 4.0x4.0 cm. In Group 2 (G2),
we performed a partial cystectomy and bladder augmentation with anionic
collagen membrane, with the same dimension (Figure-2). In Group 3 (G3)
we have performed only cystometric evaluation.
All rabbits were submitted to cystometric
study in the moment of bladder augmentation, and every four weeks until
sacrifice (M3). M0: bladder augmentation; M1: 4 weeks; M2: 8 weeks; and
M3: 12 weeks. Cystometric study was performed with awaken animals, which
were submitted to urethral dual-lumen (8F) and retal catheterism (8F).
After the drainage of vesical content, saline solution was infused at
room temperature, with continuous fill rate of 2mL/min by infusion-pump
through the proximal lumen of the urethral catheter. Vesical, detrusor,
and abdominal pressure curves were continuously registered during procedure,
by a pressure transducer connected to distal lumen of the urethral catheter.
Maximum bladder capacity (BC) was determined in the moment of urine leakage
around the catheter, and registered in an urodynamics device - Dantec™
(Duet). At M3, after cystometric study, anesthesia was performed and we
proceeded to explore the abdomen. Subsequently, we proceeded to intravesical
instillation of 10% formaldehyde and, afterwards, to total cystectomy
and bilateral nephrouretectomy. The animals were sacrificed with 3,5%
pentobarbital sodium 3mL/kg by parenteral route. After 15 days, we proceeded
to bladder section, obtaining serial longitudinal sections, spaced in
about 0,5cm. At the histopathological study using hematoxylin and eosin
(HE), at least 8 segments per bladder were evaluated. The tissue segments
were evaluated for presence or absence of implant, fibrosis, inflammatory
process, foreign body reaction, calcification, and other alterations.
For descriptive and statistical analysis
of variables, we used mean, standard deviation, and analysis of variance
(ANOVA) with 2 factors for repeated measures; Friedman and Newman-Kuels
tests. For all analysis performed the results were considered significant
when p<0.05 (12).
RESULTS
At
macroscopic evaluation (inspection) we observed, at M0, the presence of
calcium concretions adhered to chromic catgut suture, within the bladder,
in most of the rabbits. At the moment of sacrifice (M3) neither urinary
lithiasis, nor membrane extrusion was observed.
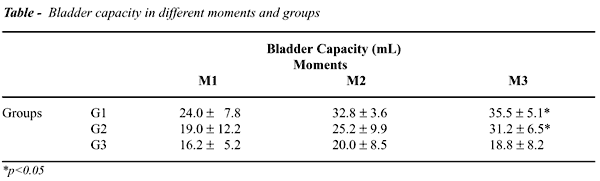
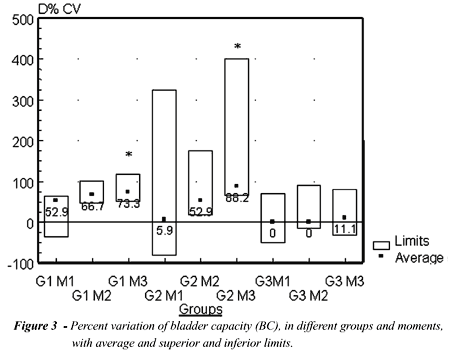
BC at M0 was 17.5±6.1mL, with no
significant difference among the groups (p=0.62). We have observed a significant
elevation only in moment 3 (M3) for Groups G1 and G2, when we analyzed
absolute values of bladder capacity (p<0.05) (Table). Evaluating percent
variations of BC related to M0, we have observed within the groups, significant
elevations in G1 and G2 at M3 (p<0.05). When we compared the groups,
we observed that between G1 and G2 there was no significant difference
at various moments. Both G1 and G2 presented significant difference when
compared to G3 at M3 (p<0.01) (Figure-3).
When we evaluate microscopic findings in
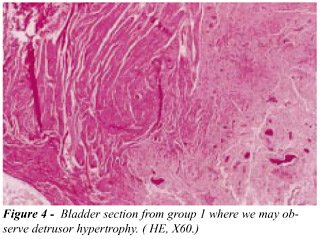
bladder, at G1 we observed the presence of a chronic inflammatory reaction,
like a foreign body reaction: a lymphoplasmocyte infiltrate in bladder
submucosa, muscular wall hypertrophy in 60% of the cases, and presence
of scarring tissue (Figure-4). At G2, we have observed absence of inflammatory
process, apparently normal muscular wall, and some regions of scarring
at the wall (Figure-5). In rabbits at G3, we observed absence of inflammation
and scarring tissue. In one rabbit we have seen glandular cystitis, and
in another one an atrophic urothelium. At observation the kidneys presented
few significant alterations: two cases of mild chronic pyelonephritis,
one at G1 and the other at G2, and one case of focal acute tubular necrosis
(G3).

DISCUSSION
Acellular
matrices are prepared from bowel, stomach or bladder, through mechanical
or chemical manipulation, in order to extract cells from the tissue, maintaining
the membrane of extracellular homogeneous matrix. Studies with these materials
implanted in bladder have shown excellent results, with total epithelization
in 4 days, and evidence of muscular and vascular regeneration in two weeks
(13). However, some complications can also be observed, as lithiasis formation
in up to 63% of the cases (14).
In our experiment, we have done bladder
augmentation with ACM with and without preserved urothelium. We used the
group with preserved urothelium to evaluate possible differences in calcification
of the material, or bladder calculi formation, for it is well know that
this animal’s urine has alkaline pH, which favors salt precipitation
in the presence of foreign bodies, with greater risk of developing bladder
calculi or graft calcification (15). We did not observe such complication,
even when ACM has remained in direct contact with urine.
We have to perform some specific studies
to evaluate whether the material presents or not less lithogenic potential.
We know that the main difference that distinguishes it from other collagen
derivatives is that this anionic membrane presents negatively charged
molecules in neutral pH, due to selective hydrolysis of the carboxyamide
groups of asparagine and glutamine, which supplies improvements in dielectric
properties, compared to native collagen (10).
Another finding with ACM use was an increase
in BC observed at the final moment (M3) in the groups augmented. Our results
were equivalent to those obtained with other materials. A study performing
only auto-augmentation in retracted bladders, with formalin, showed an
increase in BC from 15 mL to 34 mL (16). Our animals presented mean increases
from 17 to 35 mL, and this final result may be considered as the normal
mean value of a rabbit’s bladder. Another study evaluating BC of
normal rabbits, with body weights similar to our rabbits, observed that
these presented mean volumes between 33 and 37 mL (17).
In our experiment we have also tried to
evaluate the presence of tissue regeneration. After 3 months we did not
find traces of the primitive membrane, substituted by epithelium, and
scarring tissue and normal muscle. What impressed us was that the group
in which we have preserved bladder mucosa presented an intense inflammatory
process in submucosa region. Other authors that augment bladder with bowel
loops and preserve bladder mucosa did not report this alteration (18).
Atala et al. (19) used acelullar collagen
membrane graft in bladder augmentation, and they observed at histological
studies normal cell organization in augmented bladder, with epithelium,
submucosa, and muscle neoformation. A relevant fact is the occurrence
of greater bladder capacity in animals receiving graft with seeded cells.
Other authors using bladder acellular collagen membrane have not only
confirmed regeneration of bladder muscle, as also regeneration of innervation,
as confirmed by electric stimulation as well as muscarinic receptors (20).
In the literature, several authors consider that the state-of-the-art
graft should be biodegradable, i.e., should be completely eliminated after
acting as a backbone to bladder tissue regeneration. It should neither
be rejected by the host, nor promote adherences or mutagenic alterations,
and should be readily available at low cost. Anionic collagen membrane
is biodegradable, and in our study it has turned possible bladder tissue
regeneration. It is important to report that this experiment was performed
in normal bladder, and further studies will be necessary to evaluate the
responses in pathological bladders.
CONCLUSIONS
With our study we could to conclude that collagen anionic matrix showed effectiveness for bladder augmentation, demonstrating no significant difference in BC when bladder augmentation was performed with or without urothelium preservation. Bladders with urothelial preservation presented extensive inflammatory process, and we did not observe lithiasis formation with the use of the collagen anionic matrix.
REFERENCES
- Linder A, Leach GE, Raz S: Augmentation cistoplasty in the treatment of neurogenic bladder dysfunction. J Urol. 1983; 129: 491-3.
- Duel BP, Gonzalez R, Barthold JS: Alternative techniques for augmentation cystoplasty. J Urol. 1998; 159: 998-1005.
- Skinner DG, Boyd D, Lieskowsky G: Clinical experience with the Kock continent ileal reservoir for urinary diversion. J Urol. 1984; 132: 1101-7.
- Khoury JM, Timmons SL, Corbel L, Webster GD: Complications of enterocystoplasty. Urology 1992; 40: 9-14.
- McDougal WS: Metabolic complications of urinary intestinal diversion. J Urol. 1992; 147: 1199-1208.
- Gleeson M, Griffth DP: The use of alloplastic biomaterials in bladder substitution. J Urol. 1992; 148: 1377-82.
- Novick AC, Straffon RA, Banowsky LH, Nose Y, Levin H, Stewart BH: Experimental bladder substitution using biodegradable graft of natural tissue. Urology 1977; 10: 118-27.
- Atala A, Freeman MR, Vacanti JP, Shepard J, Retik AB: Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J Urol. 1993; 150: 608-12.
- Kropp BP, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, Thor KB: Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol.1996; 155: 2098-2104.
- Goissis G, Marcantonio EJr, Marcantonio RA, Lia RC, Cancian DC, de Carvalho WM: Biocompability studies of anionic collagen membranes with different degree of glutaraldehyde cross-linking. Biomaterials 1999; 20: 27-34.
- Cartwrigth PC, Snow BW: Bladder autoaugmentation: early clinical experience. J Urol. 1989, 142: 505-8.
- Morrison DF: Multivariate Statistical Methods. New York Mc Graw Hill 1967; p. 338.
- Sutherland RS, Baskin LS, Hayward SW, Cunha GR: Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol. 1996; 156: 571-7.
- Vaught JD, Kropp BP, Sawyer BD, Rippy MK, Badylak SF, Shannon HE, Thor KB: Detrusor regeneration in the rat using porcine small intestinal submucosal grafts: functional innervation and receptor expression. J Urol. 1996; 155: 374-8.
- Rosen MA, McAninch JW: Wound Closures and Sutures Techniques in Reconstrutive Procedures. In: McAninch JW: Traumatic and Reconstrutive Urology. Philadelphia,WB Saunders, 1996; pp 49-69.
- Taneli C, Genc A: Long-term follow-up and elevation of autoaugmentation cystoplasty in an animal model. Int Urol Nephrol. 1999; 31: 55-9.
- Amaro JL, Curi PR, Fabris VH, Trindade JCS: Ampliação vesical com dura-máter e pericárdio bovino. Estudo comparativo em coelhos. Braz J Urol. 1997; 23: 88-92.
- Nguyen DH, Mitchell ME, Horowitz M, Bagli DJ, Carr MC: Demucosalized augmentation gastrocystoplasty with bladder autoaugmentation in pediatric patients. J Urol. 1996;156: 206-209.
- Atala A, Yoo JJ: Allogenic bladder submucosa as a new biomaterial for bladder augmentation. J Urol. 1997; 157: 234A.
- Piechota HJ, Dahms SE, Nunes LS, Dahiya R, Lue TE, Tanagho EA: In vitro functional properties of the rat bladder regenerated by the bladder acellular matrix graft. J Urol. 1998; 159: 1717-24.
_______________________
Received: January 28, 2002
Accepted after revision: May 27, 2002
_______________________
Correspondence address:
Dr. Fábio Gustavo Oliveira Lepper
Rua Coronel Manoel Luiz dos Santos, 465 / 24
Botucatu, SP, 18.603-310, Brazil
Fax: + 55 14 6822-0421
E-mail: jcarlos@fmb.unesp.br
EDITORIAL COMMENT
The
drawbacks associated with current augmentation modalities have created
an interest in developing better materials for bladder augmentation. Nowadays,
investigators are attempting to engineer virtually every human tissue.
The first stage of tissue engineering begins with the design and fabrication
of a porous scaffold. This scaffold serves as a three-dimensional template
for initial cell attachment and subsequent tissue formation. The authors
have applied the principle of in vivo tissue-engineering strategies. They
have used an anionic collagen membrane, which was elaborated from collagen
gels prepared by the treatment of purified bovine serosa and tendon (reference
10 in the article).
Currently, numerous investigators have stated
that better outcomes with the use of grafts for bladder augmentation can
be obtained with the use of acellular matrices elaborated from small intestinal
submucosa (SIS) or from bladder allograft (BAMA) (1). The assumption is
that the appropriate extracellular environment provided by a scaffold,
which contains almost all major components of the native extracellular
matrix, is sufficient to effectively organize the regenerative capacity
of the various components of the bladder wall. The efficacy of acellular
matrix depends on its low antigenicity, its capacity for rapid vascularization,
and its stability as a bladder template. These properties will be determined
largely by the final composition of the acellular matrix. In this way,
the authors’ collagen membrane seems more appropriate to be used
in other conditions than bladder augmentation.
Regardless of the strategy employed to develop
a better bladder wall substitute, the incorporation of an acellular bladder
augment involves 2 processes; one from the edge of the defect, and one
from islands of cells in the midst of the defect. As the regeneration
from the edges is more expressive than islands of cells, less encouraging
outcomes at longer time are expected, if one uses a large patch (1). The
authors have shown that the preservation of urothelium did not provide
better results than the classical patch augmentation. However, the range
of the bladder capacity was too wide in the group that collagen membrane
was performed exclusively. Furthermore, the authors presented good short-term
outcomes, but I am concerned about the efficacy of the anionic collagen
membrane as a bladder substitute at long-term follow-up.
The challenge for more advanced scaffold
systems is to arrange cells/tissue in an appropriate 3D configuration,
and present molecular signals in an appropriate spatial and temporal fashion,
so that the individual cells will grow and form the desired tissue structures
– and will do so in a way that can be reproduced at low costs and
on a large scale (2).
References
1. Brown
AL, Fahrt W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA: 22 week
assessment of bladder acellular matrix as a bladder augmentation material
in a porcine model. Biomaterials 2002; 23:2179-90.
2. Hutmacher DW: Scaffold design and fabrication technologies for tissue
engineering tissues – state of the art and future perspectives. J
Biomater Sci Polymer Edn. 2001, 12:107-24.
Dr. E. Alexsandro da Silva
Urogenital Research Unit
State University of Rio de Janeiro
Rio de Janeiro, RJ, Brazil