MINIMALLY
INVASIVE PROCEDURES FOR URETHRAL INCONTINENCE: IS THERE A ROLE FOR LAPAROSCOPY?
(
Download pdf )
OMID ROFEIM(1), PAULOS YOHANNES(2), GOPAL H. BADLANI(1)
(1)Department of Urology, Long Island Jewish Medical Center, New Hyde Park, New York, and (2)Division of Urology, Creighton University, Omaha, Nebraska, USA
ABSTRACT
This
article focuses on the minimally invasive surgical approaches for the
treatment of stress urinary incontinence (SUI). The role of laparoscopic
suspension is reviewed and compared with other minimally invasive techniques,
such as the pubovaginal sling procedure and injection of the urethral
bulking agents.
The role of laparoscopic Burch colposuspension
remains ill defined in 2002. Once this minimally invasive technique is
shown to duplicate the success rate of the open Burch procedure, it could
be offered as a first-line therapy to patients with SUI. At this time,
the pubovaginal sling (PVS) offers the best long-term results with acceptable
low complication rates of urinary retention, urgency, and sling erosion
or infection. These complications are rarely seen with the laparoscopic
repair but the incidence of bladder injuries is higher. The PVS operation
can be performed as a salvage procedure, in obese patients, and concomitant
with cystocele and rectocele repair whereas data for laparoscopy in these
conditions are lacking. Until the long-term efficacy of the laparoscopic
repair is clearly defined, offering it to patients as a minimally invasive
therapy denies them of procedures with superior efficacy.
Key words:
stress urinary incontinence; surgical treatment; laparoscopy, prostheses
and implants
Int Braz J Urol. 2002; 28: 403-12
INTRODUCTION
In
the United States, 15 billion dollars are spent annually for the treatment
of urinary incontinence (1). The prevalence of incontinence is 20% in
women older than 40 years old (2), 40% in ambulatory elderly women (3),
and up to 50% in nursing home residents (4). Unfortunately, less than
half of the patients with incontinence discuss their condition with health
care providers (5).
Incontinence is the involuntary loss of
urine. It may be due to bladder abnormalities, such as detrusor overactivity,
or urethral dysfunction. The 2 types of urethral dysfunction are urethral
hypermobility and intrinsic sphincter deficiency (ISD). The weakness of
pelvic floor support with resulting rotational descent of the bladder
neck and proximal urethra during Valsalva maneuvers are the main causes
of incontinence in women with hypermobility. ISD is characterized by decreased
urethral resistance due to lack of internal sphincter mechanism. Neurological
conditions, previous pelvic surgery, hypoestrogenic states, and aging
process are some of the causes of ISD.
Urethral continence is believed to be multifactorial,
including tone and contraction of smooth and striated muscles, viscoelastic
properties of extracellular matrix (proteoglycans, glycopreoteins, collagen,
and elastin), structural support of the posterior urethra, transmission
of abdominal pressure to the bladder and urethra, apposition of urethral
lumen, and neurological control. A defect of any of the above properties
may lead to incontinence. Lack of estrogen can decrease coaptation of
the urethral lumen. Loss of structural support of posterior urethra can
also lead to urethral hypermobility and incontinence (6). Others believe
that unequal transmission of abdominal pressure to the bladder and urethra
can cause incontinence when the bladder pressure exceeds that of the urethra
(7). We have demonstrated that the endopelvic fascia and skin of women
with stress urinary incontinence secrets more elastase and collagenase
than control subjects (8). This may result in decreased amounts of extracellular
matrix of the pelvic floor leading to the development of SUI. In addition,
we showed that the increased level of the proteolytic enzymes in the skin
and plasma of women with SUI suggests a systemic process not limited to
the endopelvic fascia. Other investigators believe that there is radiological
evidence to support that the anterior and posterior walls of the bladder
neck and proximal urethra pull apart from each other with increased abdominal
pressure leading to incontinence (9).
A multitude of surgical and non-surgical
treatment modalities has been described to correct SUI. In the published
review of the American Urological Association Guidelines, the open Burch
and sling procedures had the best results up to 48 months of follow-up
(10). Minimally invasive approaches to correct SUI have followed the path
from open surgery to needle suspension to bioinjectibles and to possible
drug therapy. The needle suspension procedure while initially popular,
failed in the long-term follow-up (11). Minimally invasive modifications
of the pubovaginal sling and the laparoscopic approach to replace Burch
colposuspension are reviewed here for the current state of the art.
PUBOVAGINAL SLING
The
gold standard of treatment for SUI due to ISD is the pubovaginal sling
(PVS) procedure. Von Giordano was the first person to describe the procedure
in 1907 (12). In 1910, Goebell was the first to use the pyramidalis muscle
(13). Many modifications were introduced, but PVS lost popularity due
to extensive retropubic dissections and complications. McGuire & Lytton
reintroduced the PVS in 1978 using autologous rectus fascia (14). In that
series, at a mean follow-up of 2.3 years 41 out of 52 patients (80%) were
cured with operation alone and another 11% were cured with operation and
medication. Blaivas & Jacobs later modified the procedure in 1991
and an overall success rate of 91% was reported (15). However, the PVS
was performed as a salvage procedure after other continence procedures
had failed. Now, the PVS procedure is indicated as the primary treatment
of incontinence due to ISD. It can be performed under general or regional
anesthesia, in less than 2 hours, and as an ambulatory or overnight stay
basis.
The choice of sling material includes autologous,
allograft, and synthetic materials. The rectus fascia, fascia lata, vaginal
wall, and a number of other tissues have been used as autologous sling
material. Harvesting of fascia lata requires a separate thigh incision
but larger strips of more uniform fascia can be obtained compared to rectus
fascia, especially if patient had previous abdominal surgery. Cure rates
of up to 98% have been reported using fascia lata (16). Anterior vaginal
wall slings have achieved 90-94% cure rates with a mean follow-up of 24
months; however; longer follow-up is lacking (17,18). Autologous fascia
is less costly and less prone to infection and erosion than other material,
however, larger or separate incisions, longer operative time, and more
post-operative pain are observed when autologous fascia is used. Use of
cadaveric fascia lata as sling material was first reported in 1996 (19).
Long-term safety and efficacy of allografts have been well documented
in the orthopedic literature (20). The risk of HIV transmission from allografts
is estimated to be 1 in 1,667,600 (21). A recent study demonstrated that
intact DNA was present in freeze-dried, gamma-irradiated cadaveric fascia
lata and acellular cadaveric dermis (22). However, the infectious potential
of this finding remains unknown. Allografts are available in different
sizes and eliminate the need for harvesting. Similar continence rates
have been achieved with autologous and allograft material but the operative
time, post-operative pain, and hospital stay have been significantly shorter
when allografts are used (23,24).
Some of the synthetic sling materials include
polyethylene (Mersiline), polytetrafluoroethylene (Gore-Tex), polypropylene
(Marlex), polyester with bovine collagen matrix (ProteGen), Teflon, and
Silastic. Similar to allografts, synthetic materials decrease operative
time and eliminate the need for tissue harvesting. In addition, they cannot
be degraded by enzymatic reactions (25). Earlier series reported high
rate of erosions, infections, and sling removal (26,27). A cure rate of
82% was reported in those series. In a recent prospective study, an antimicrobial
mesh was compared with vaginal wall sling. At a mean follow-up of 22 months,
SUI was cured in 95% of the mesh group and 70% of those with vaginal wall
sling. De novo urge incontinence developed in 12.5% of the mesh and 14.3%
of the vaginal wall sling group. No tissue erosions or infections were
reported (28). In another investigation, 94% cure rate was reported after
a minimum of 2 years of follow-up using autologous or synthetic material
and a bone-anchoring system to support sutures to the pelvic bone (29).
We use a polypropylene mesh sling with bone anchors. Report of our preliminary
results showed that 91.4% of the patients were dry at a mean follow-up
8.4 months without any infections or erosions (30). When these patients
were followed-up for a mean of 52 months (longest 66 months), 70% of the
50 patients were completely dry, 20% rarely leaked urine, 2% leaked a
moderate amount, and 8% failed the procedure. No infections or erosions
occurred but bone anchors were removed in one patient due to pain (unpublished
data).
In 1996, Ulmsten et al. reported the initial
experience with tension-free vaginal tape (TVT) procedure (31). A polypropylene
mesh is placed at the level of mid-urethra through a small vaginal incision
under local anesthesia as an outpatient procedure. The longest follow-up
result reported showed that at a median follow-up of 56 months, out of
90 patients 85% were cured, 11% were significantly improved, and 4% failed
(32). Similar results were reported after a mean follow-up of 4 years
when TVT procedure was performed for ISD. Seventy four percent were cured,
12% were improved, and 14% failed. Failure was more common in those with
leak point pressure of < 10 cm H2O (33). Other investigators have also
shown that TVT may not be as effective in those with ISD. In a study of
319 patients in which 43 (13%) had urethral pressure of < 20 cm H2O,
post-operative leakage after a median follow-up of 7 months was significantly
more than those with urethral leak pressure of > 20 cm H2O. However,
patient satisfaction was the same between the 2 groups (34). Another prospective,
multi-center study demonstrated that after 2 years of follow-up, objective
continence rate was 37% for patients with ISD and 95% for those with SUI
types I and II (p = 0.0006) after TVT procedure. However, subjective evaluation
did not reveal any differences in continence rates (35). It was assumed
that the position of the tape at the mid-urethral level (not the bladder
neck) might be the cause of failure to restore continence. Therefore,
patients with ISD should be informed regarding the lower success rate
of TVT prior to the procedure.
The main complications of TVT procedure
are voiding difficulty, bladder perforation, and de novo urgency. A large
study showed that urinary retention occurred in 2.8% (17 out of 600 patients)
lasting more than one week post-operatively. All 17 patients underwent
transvaginal release of TVT and 16 remained dry after release (36). Bladder
perforation and de novo urge incontinence occur in 6-11% and 25%, respectively
(37,38).
In summary, the PVS procedure shows excellent
long-term success rate for the treatment of SUI. Urinary retention, de
novo urgency, and a small risk of erosion and infection remain as complications
of this procedure. However, the PVS should be the standard against which
all other minimally invasive therapies for incontinence are examined.
LAPAROSCOPIC BURCH COLPOSUSPENSION
Although
numerous treatment options are available for patients with SUI, the open
Burch procedure has stood the test of time (39-41). Vaginal approaches,
on the other hand, continue to undergo a series of modifications in search
for the most durable, biocompatible support material. As a natural extension
of the success of laparoscopy in other areas, laparoscopic Burch colposuspension
was introduced by Vancaille & Scheussler in 1991 to provide patients
with an alternative treatment option associated with less morbidity (42).
Laparoscopic pelvic surgery provides better visualization, shorter hospital
stay, better cosmetics, less postoperative pain, and faster recovery to
normal daily activity. However, despite the renewed interest in the application
of laparoscopic technique in the management of SUI, a dichotomy of opinion
remains regarding its long-term efficacy. Laparoscopic colposuspension
is historically regarded as having good, short-term success rate of over
90% (43-48) but this rate declines with longer follow-up to 59%-68% (Table-1)
(49-50). This is in contrast to the open Burch procedure, which is associated
with a 10-year success rate of at least 81.6% (51). Although the laparoscopic
approach is arguably more cost-effective and less morbid than the open
procedure originally described by Burch in 1961, laparoscopic Burch is
not recommended for recurrent SUI (40,52-54). The wide range of success
rates reported by some of the most skilled laparoscopists has led many
to scrutinize this technique. This may be partly related to the difference
in the definition of success rate after incontinence surgery, limited
follow-up, and lack of standardized suturing technique.
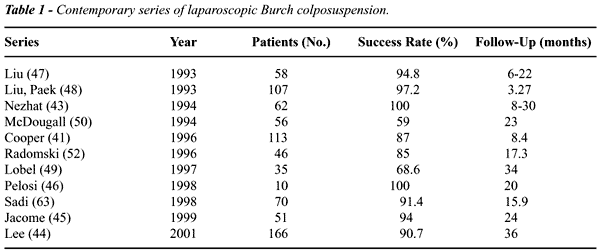
Laparoscopic Burch colposuspension has been
described using the transperitoneal or extraperitoneal approach, using
3 to 5 trocars. The extraperitoneal route is favored by most authors (40,52,55,56)
and is similar to the technique described by Burch (39). In this approach,
the space of Retzius is rapidly dissected using a balloon, or without
a balloon by finger and pneumodissection with CO2 (40,43). This in turn
reduces the operative time, and helps minimize the cost (40,52). The extraperitoneal
approach also avoids intraperitoneal pelvic adhesions, minimizes the risk
of intra-abdominal injury, and is associated with a shorter learning curve.
The main disadvantage of extraperitoneal laparoscopic colposuspension
is the risk of increased absorption of CO2 leading to pneumomediastinum
and pneumothorax (41,57). On the other hand, the transperitoneal approach
is suitable for patients undergoing concomitant pelvic surgery (47-49,58,59).
The operative time with this technique may be prolonged due to the need
to take down adhesions, mobilize the bladder, and difficulty in retracting
intra-abdominal organs. The gasless approach has also been described (60).
A pilot study by Flax has shown the gasless approach to be feasible and
easier than the traditional approach leading to lower conversion rates,
simpler suture tying, and decreased operative time.
One of the factors that affects the learning
curve and determines the success rate of laparoscopic colposuspension,
is the intuition one has to develop in determining suture tension while
approximating the Cooper’s ligament to the pubocervical fascia. Because
of the relative lack of tactile feedback with laparoscopic surgery, the
technique warrants that the urologist must overcome this portion of the
learning curve outside the operating room. Tying of the knots can be performed
with intracorporeal free-hand technique, using the Endostitch device (US
Surgical Corporation, Norwalk, CT, USA), or by using an extracorporeal
knot pusher (48). The type of suture used to elevate the bladder neck
also varies. Although Burch proposed an absorbable suture in his initial
report, some have used non-absorbable sutures to minimize recurrence (41,58).
The use of curved needle, straight needle, and Stamey’s needle has
been described with laparoscopic Burch colposuspension (49). Broken needles
at the time of laparoscopy, though rarely reported, can be very frustrating
(61). In all cases, however, emphasis is placed on the degree of tension
placed on the suture rather than the type of needle or suture utilized.
Finally, the number of sutures placed on
each side of the urethra has been studied in a prospective, randomized
study by Persson & Wolner-Hanssen (62). One hundred and sixty-one
women were randomized to receive one (78) or 2 (83) sutures. At one-year
follow-up, the objective cure rate was 83% for the two-suture group. Therefore,
placement of 2 sutures at the bladder neck is recommended.
There have been numerous reports confirming
the feasibility of laparoscopic Burch colposuspension (41,43-47,49,52,63).
Review of 10 series (1993-2001) shows that the laparoscopic approach is
associated with less postoperative analgesic use, shorter hospital stay,
and rapid recovery (Table-2). However, durable long-term results that
compare with the open retropubic technique have yet to be demonstrated.
Comparative studies between the open and laparoscopic approach have been
reported (55,58,64). Miannay and associates reported on an age, stage,
and associated procedures-matched retrospective analysis of 72 patients
(58). With a mean follow-up of 17 and 46 months for the laparoscopic and
open groups, respectively, the cure rate after one and 2 years was similar
in both groups. Similarly, Saidi et al. (55) retrospectively compared
laparoscopic colposuspension with open Burch in 157 patients. The short-term
cure rate at 12-16 months was comparable to the open procedure (91.4%
vs. 91.8%), and complication rate was lower for the laparoscopic group
(15.8% vs. 33.3%). On the other hand, reports by McDougall & Portis
on 56 patients with SUI have demonstrated poor outcome with this procedure.
At an average follow-up of 23 months, the success rate was only 59%. If
preoperative abdominal leak point pressure was less than 90 mmHg, the
success rate was 25% after 30 months of follow-up (50).
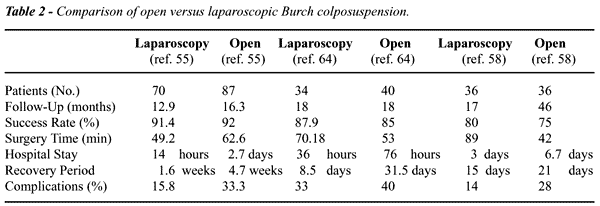
The only randomized, prospective study comparing
open Burch to the laparoscopic approach found a lower success rate with
the laparoscopic approach, which was statistically significant (65). Most
recently, Brenner reported his experience with 36 laparoscopic Burch colposuspensions
and 42 suburethral sling procedures (59). The Burch procedure was for
primary incontinence while the suburethral sling was done for secondary
cases. Although follow-up was limited (15 months for laparoscopy and 11
months for sling), the sling group had higher success rate than the laparoscopic
group (93% vs. 83%).
The complication rate related to the laparoscopic
approach is higher than the open procedure (5-8% vs. 8-22%) (66). The
most common intraoperative complication is lower urinary tract injury.
Bladder injury, which occurs at an incidence of 2.17-18%, is common in
patients with prior pelvic surgery (40,41,48,55,59,66,67). Bladder catheter
drainage during surgery and meticulous dissection help prevent most bladder
injuries. In the majority of cases, these injuries can be managed laparoscopically
obviating the need to convert to an open procedure (52). Conversion rates,
especially in the earlier stages of learning, can be as high as 26% (52).
Rare cases of partial ureteral obstruction have been reported (48,68).
The development of overactive bladder after laparoscopic Burch colposuspension
is a well-recognized phenomenon (40,41,43,48,58,64,69). It occurs at an
incidence of 2.8%-8% and has been attributed to extensive dissection of
the bladder (43,48,69). The high incidence of rectocele (11-30%) and enterocele
(1-5.7%) has led many to obliterate the cul-de-sac, and perform enterocele
and rectocele repair, as well as vaginal wall suspension at the time of
colposuspension (40,43,48,49). Furthermore, the incidence of postoperative
permanent or transient urinary retention is low (1.8%) (48). Granulation
tissue at the vagina from suture protruding through vaginal mucosa, and
small bowel obstruction through a peritoneal defect have been reported
as complications of the laparoscopic approach (49,70). Osteitis pubis
has not been reported with the laparoscopic Burch procedure (71).
URETHRAL BULKING AGENTS
Periurethral
bulking agents are alternative forms of minimally invasive therapy for
urinary incontinence due to ISD. The bulking agents serve to increase
the coaptation of the urethral mucosa and help prevent involuntary loss
of urine during periods of increased abdominal pressure. Delivery of the
bulking agent can be accomplished via the transurethral or periurethral
route under local anesthesia and as an outpatient procedure (72). Most
studies evaluating the efficacy of bulking agents for the treatment of
ISD have demonstrated that the success rate drops after 6 months due to
distant migration or local degradation of the bioinjectable particles.
Currently, there are two Food and Drug Administration (FDA)-approved periurethral
bulking agents in the United States, collagen and Durasphere (Carbon Medical
Technologies, Inc., St. Paul, Minnesota, USA). Experience with Teflon
as an injectable agent, as well as Durasphere, has been disappointing
due to reported cases of particle migration (73). Durasphere is a carbon-coated
bead that was approved by FDA in 1999 for the treatment of incontinence
due to ISD. The success rate associated with this agent is limited. Pannek
at al. have recently reported their experience in 7 men and 11 women with
ISD (73). Their results demonstrated that the success rate drops from
76% at 6 months to 33% at 12 months. Furthermore, at 6 months, migration
of the beads was noted into the distant lymph nodes and urethral mucosa.
More recently, Lightner et al. have reported the only multi-center, randomized,
controlled, double-blind study comparing Durasphere to bovine collagen
in the treatment of ISD (74). In this study, an average of 4.83 ml of
Durasphere and 6.23 ml of collagen were injected. At 12 months of follow-up,
the 2 agents produced similar results in terms of improving incontinence.
Improvement rates with Durasphere and bovine collagen at 12 months were
80.3% and 69.1% (p = 0.162), respectively. Currently, experience with
Durasphere is limited and more investigation is required before offering
it to patients with ISD as the first line of therapy.
The use of collagen for the treatment of
ISD has gained widespread popularity since it was FDA-approved in 1993
(72,75-78). Contigen (C.R. Bard, Covington, GA, USA) has been demonstrated
to be safe, durable, and efficacious. The reported success rate with injectable
collagen varies from 88% - 100%. Like all the injectable agents, the success
rate declines with longer follow-up (13%) (75). Richardson et al. reported
their results with collagen for the treatment of ISD in 42 women (78).
The mean amount of collagen injected per patients was 28.3 ml. The greatest
improvement in incontinence was noted after 17.2 ml was injected. At a
mean follow-up of 42 months, 83% were greatly improved. Similarly, Cross
et al. reported their experience with collagen in 139 women with ISD (77).
Seventy-two percent of patients improved after 2 or fewer injections,
whereas 11% required booster injections more than 6 months after the initial
treatment. Complications, in this series, were rare. Elsergany et al.
investigated the relationship between the grade of incontinence and success
of injection and found no difference between the 2 factors (76). At a
mean follow up of 18 months, an overall success rate of 81.8% was reported.
Finally, Groutz et al. showed that using strict criteria the cure rate
of collagen injection was only 13% (75). Clearly, the short-term success
rate with collagen is favorable, and overall morbidity is low. Uncommon
complications due to collagen injection include formation of urethral
diverticulum, permanent urinary retention, abscess formation, delayed
hypersensitivity, and systemic arthralgia (79-82).
The use of autologous fat is an attractive
treatment option. Fat may be readily harvested by liposuction from the
abdomen or thigh. Autologous fat has not been shown to be more efficacious
than the other bulking agents (83,84). Comparative studies evaluating
collagen and autologous fat have demonstrated that autologous fat is not
as efficacious and durable as collagen in improving urinary incontinence
(85). Although the use of autologous fat may be cost-effective, it requires
numerous injections to sustain continence. The possibility of pulmonary
fat embolism has made this agent less popular (82).
CONCLUSIONS
The role of laparoscopic Burch colposuspension remains ill defined in 2002. Most authors echo the need for more prospective, multi-center, randomized studies comparing open to laparoscopic Burch colposuspension to better define the role of laparoscopy in the management of SUI. More standardized suturing techniques and methods of measuring the suture tension intraoperatively will contribute to better results. Once this minimally invasive technique is shown to duplicate the success rate of the open Burch procedure, it could be offered as a first-line therapy to patients with SUI. At this time, the PVS offers the best long-term results with acceptable low complication rates of urinary retention, urgency, and sling erosion or infection. These complications are rarely seen with the laparoscopic repair but the incidence of bladder injuries is higher. The PVS operation can be performed as a salvage procedure, in obese patients, and concomitant with cystocele and rectocele repair whereas data for laparoscopy in these conditions are lacking. Until the long-term efficacy of the laparoscopic repair is clearly defined, offering it to patients as a minimally invasive therapy denies them of procedures with superior efficacy.
REFERENCES
- Fantl JA, Newman DK, Colling J: Urinary Incontinence in Adults: Acute and Chronic Management. Clinical Practice Guideline No. 2, 1996 Update. Rockville, Maryland: US Department of Health and Human Services, Agency for Health Care Policy and Research. 1992; AHCPR Pub. No. 96-0682.
- Perry S, Shaw C, Assassa P, Dalloso H, William K, Brittain KR, et al.: An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med. 2000; 22: 427-34.
- Weinberger MW, Goodman BM, Carnes M: Long-term efficacy of nonsurgical urinary incontinence treatment in elderly women. J Genrontol A Biol Sci Med Sci. 1999; 54: M117-21.
- National Institute of Health Consensus Development Conference on Urinary Incontinence in Adults. Bethesda, Maryland, October 3-5, 1988. Proceedings. 1990; J Am Geriatr Soc, 38: 263-386.
- Burgio KL, Ives DG, Locher JL, Arena VC, Kuller LH: Treatment seeking for urinary incontinence in older adults. J Am Geriatr Soc, 42: 208-212, 1994.
- Walters MD, Jackson GM: Urethral mobility and its relationship to stress incontinence in women. J Repro Med. 1990; 35: 777-84.
- Rosenzweig BA, Bhatia NN, Nelson AL: Dynamic urethral pressure profile transmission ration: What do the numbers mean? Obst Gynecol. 1991; 77: 586-90.
- Mathrubutham M, Maytal A, Rao SK, Badlani GH, Kushner L: Elastolytic and collagenolytic activity is elevated in conditioned media from skin and endopelvic fascia explants of women with pelvic floor weakening. J Urol. 2000; 163 (Suppl. 95), Abstract 415.
- Mostwin JL, Yang A, Sanders R, Genadry R: Radiography, sonography, and magnetic resonance imaging for stress incontinence. Urol Clin North Am. 1995; 22: 539-49.
- Leach GE, Dmochowski RR, Appell RA, Blaivas JG, Hadley HR, Luber KM, et al.: Female Stress Urinary Incontinence Clinical Guidelines Panel Summary Report on Surgical Management of Female Stress Urinary Incontinence. The American Urological Association. J Urol. 1997; 158 (3 Pt 1): 875-80.
- Trockman BA, Leach GA: Needle suspension procedures: past, present, and future. J Endourol. 1996; 10: 217-20.
- Ridley JH: The Goebll-Stoeckel Sling Operation. In: Mattingly RF, Thompson JD (ed.). TeLinde’s Operative Gynecology. Philadelphia, Lippincott, 1985.
- Goebell R: Zur operativen besertigung der angeborenen incontinentia vesicae. Z.f.Gynak. 1910; 2: 187.
- McGuire EJ, Lytton B: Pubovaginal sling for stress incontinence. J Urol. 1978; 119: 82-4.
- Blaivas JG, Jacobs BZ: Pubovaginal fascial sling for the treatment of complicated stress urinary incontinence. J Urol. 1991. 145: 1214-8.
- Beck RR, McCormick S, Nordstrom L: The fascia lata sling procedure for treating recurrent genuine stress incintinene of urine. Obstet Gynecol. 1988; 72: 699-703.
- Raz S, Siegel AL, Short JL, Snyder JA, Snyder JA: Vaginal wall sling. J Urol. 1989; 14: 43-6.
- Juma S, Little NA, Raz S: Vaginal wall sling: four years later. Urol. 1992; 39: 424-8.
- Handa VL, Jenson JK, Germain MM, Ostergard DR: Banked human fascia lata for the suburethral sling procedure: a preliminary report. Am J Obstet Gynecol. 1996; 88: 1045-49.
- Cooper JL, Beck CL: History of soft tissue all grafts in orthopedics. Sport Med Arthroscopy Rev. 1993; 1: 2-16.
- Buck BE, Malinin TI, Brown MD: Bone transplantation and human immunodeficiency virus. An estimate of risks of acquire immunodeficiency syndrome (AIDS). Clin Ortho. 1989; 240: 129-36.
- Choe JM, Bell T: Genetic material is present in cadaveric dermis and cadaveric fascia lata. J Urol. 2001; 166: 122-4.
- Wright EJ, Iselin CE, Carr LK, Webster GD: Pubovaginal sling using cadaveric allograft fascia for the treatment of intrinsic sphincter deficiency. J Urol. 1998; 160: 759-62.
- Flynn BJ, Marinkovic SP, Yap WT: Risks and benefits of using allograft fascia lata for pubovaginal sling. J Urol. 1999; 161, Abst 1195.
- Corujo M, Badlani GH: The use of synthetic material in the treatment of women with SUI lends strength and disability. Contemp Urol. 1999. 11: 76-81.
- Stanton SL, Brindley GS, Holmes DM: Silastic sling for urethral sphincter incompetence in women. Br J Obstet Gynaecol. 1985; 92: 747-50.
- Horbach NS, Blanco JS, Ostergard DR, Bent AE, Cornella JL: A suburethral sling procedure with polytetrafluoroethylene for the treatment of genuine stress urinary incontinence in patients with low urethral closure pressure. Am J Obstet Gynecol. 1988; 71: 648-52.
- Choe JM, Ogan K, Battino BS: Antimicrobial mesh versus vaginal wall sling: a comparative outcomes analysis. J Urol. 2000; 163: 1829-34.
- Appell RA: The use of bone anchoring in the surgical treatment of female stress urinary incontinence. World J Urol. 1997, 15: 300-5.
- Hom D, Desautel MC, Lumerman JH, Feraren RE, Badlani GH: Pubovaginal sling using polypropylene mesh and Vesica bone anchors. Urol. 1998; 51: 708-13.
- Ulmsten U, Henriksson L, Johnson P, Varhos G: An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996; 7: 81-5.
- Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U: Long-term result of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12 (Suppl 2): S5-8.
- Rezapour M, Falconer C, Ulmsten U: Tension-free vaginal tape (TVT) in stress incontinent women with intrinsic sphincter deficiency (ISD) - A long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12 (Suppl 2): S12-14.
- Kulseng-Hanssen S: Success rate of TVT operation in patients with low urethral pressure. Neurourol Urodyn. 2001; 20: 417, Abst 29.
- Ohkawa A, Kondo A, Baba S, Japan TVT Trial Group: TVT operation: is it effective for those patients suffered from type III incontinence? Neurourol Urodyn. 2001; 20: 419, Abst 30.
- Klutke C, Siegel S, Carlin B, Paszkiewicz A, Klutke J: Urinary retention after tension-free vaginal tape procedure; incidence and treatment. Urol. 2001; 58: 697-701.
- Meschia M, Pifarotti P, Bernasconi F, Guercio E, Maffiolini M, Magatti F, et al.: Tension-free vaginal tape: analysis of outcomes and complications in 404 stress incontinent women. Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12 (Suppl 2): S24-7.
- Jeffry L, Deval B, Birsan A, Soriano D, Darai E: Objective and subjective cure rates after tension-free vaginal tape for treatment of urinary incontinence. Urol. 2001; 58: 702-6.
- Burch JC: Burch urethrovaginal fixation to Cooper’s ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961; 81: 281-90.
- Meltomaa S, Haarala M, Makinen J, Kiiholma P: Endoscopic colposuspension with simplified extraperitoneal approach. Tech Urol, 3: 216-221, 1997.
- Cooper MJ, Cario G, Lam A, Carlton M: A Review of results in a series of 113 laparoscopic colposuspensions. Aust N Z J Obstet Gynecol. 1996; 36: 44-8.
- Vancaille TG, Schuessler WW: Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991; 1: 169-73.
- Nezhat CH, Nezhat F, Nezhat CR, Rottenberg H: Laparoscopic retropubic cystourethropexy. J Am Assoc Gynecol Laparosc. 1994; 1 (4 Pt 1): 339-49.
- Lee CL, Yen CF, Wang CJ, Jain S, Soong YK: Extraperitoneal approach to laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 2001; 8: 374-77.
- Jacome EG, Tutera G, Mattox FT: Laparoscopic Burch urethropexy in a private clinical practice. J Am Assoc Gynecol Laparosc. 1999. 6: 39-44.
- Pelosi MA 3rd, Pelosi MA: Laparoscopic-assisted transpectineal needle suspension of the bladder neck. J Am Assoc Gynecol Laparosc. 1998; 5: 39-46.
- Liu CY: Laparoscopic retropubic colposuspension (Burch procedure): a review of 58 cases. J Reprod Med. 1993; 38: 526-30.
- Liu CY, Paek W: Laparoscopic retropubic colposuspension (Burch procedure). J Am Assoc Gynecol Laparosc. 1993; 1: 31-5.
- Lobel RW, Davis GD: Long-term results of laparoscopic Burch urethropexy. J Am Assoc Gynecol Laparosc. 1997; 4: 341-5.
- McDougall EM, Portis A: The laparoscopic bladder neck suspension fails the test of time. J Urol. 1999; 161: 105, Abst 393.
- Feyereisl J, Dreher E, Haenggi W, Zikmund J, Schneider H: Long-term results after Burch colposuspension. Am J Obstet Gynecol. 1994; 171: 647-52.
- Radomski SB, Herschorn S: Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996; 155: 515-8.
- Kohli N, Jacobs PA, Sze EH, Roat TW, Karram MM: Open compared with laparoscopic approach to Burch colposuspension: a cost analysis. Obstet Gynecol. 1997; 90: 411-5.
- Kung RC, Lie K, Lee P, Drutz HP: The cost-effectiveness of laparoscopic versus abdominal Burch procedures in women with urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1996; 3: 537-44.
- Saidi MH, Gallagher MS, Skop IP, Saidi JA, Sadler RK, Diaz KC: Extraperitoneal laparoscopic colposuspension: short-term cure rate, complications, and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998; 92: 619-21.
- Batislam E, Germiyanoglu C, Erol D: Simplification of laparoscopic extraperitoneal colposuspension: results of two-port technique. Intl Urol Nephrol. 2000; 32: 47-51.
- Wolf JS, Monk TG, McDougall EM, McClennan BZ, Clayman RV: The extraperitoneal approach and subcutaneous emphysema are associated with greater absorption of carbon dioxide during laparoscopic renal surgery. J Urol. 1995; 154: 959-63.
- Miannay E, Cosson M, Lanvin D, Querleu D, Crepin G: Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998; 79: 159-66.
- Brenner B: Comparing the laparoscopic Burch colposuspension and the suburethral sling. N Z Med J. 2001; 114: 146.
- Flax S: The gasless laparoscopic Burch bladder neck suspension: early experience. J Urol. 1996; 156: 1105-7.
- Lynch CM, Powers AK: Management of a broken needle at the time of laparoscopic Burch. JSLS. 2000; 4: 275-6.
- Persson J, Wolner-Hanssen P: Laparoscopic Burch colposuspension for stress urinary incontinence: a randomized comparison of one or two sutures on each side of the urethra. Obstet Gynecol. 2000; 95: 151-5.
- Sadi, MH, Sadler RK, Saidi JA: Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998; 5: 247-52.
- Fatthy H, El-Hao M, Samaha I, Abdallah K: Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001; 8: 99-106.
- Burton G: A randomized comparison of laparoscopic and open colposuspension. Neurourol Urodyn. 1994; 13: 497-8.
- Myers DL, Peipert JF, Rosenblatt PL, Ferland RJ, Jacobson ND: Patient satisfaction with laparoscopic Burch retropubic urethropexy. J Reprod Med. 2000; 45: 939-43.
- Speights SE, Moore RD, Miklos JR: Frequency of lower urinary tract injury at laparoscopic burch and paravaginal repair. J Am Assoc Gynecol Laparosc. 2000; 7: 515-8.
- Ferland RD, Robenblatt P: Ureteral compromise after laparoscopic Burch Colpopexy. J Am Assoc Gynecol Laparosc. 1999; 6: 217-9.
- Ou CS, Rowbotham R: Five-year follow-up of laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc, Adv Tech A. 1999; 9: 249-52.
- Margossian H, Pollard RR, Walters MD: Small bowel obstruction in a peritoneal defect after laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1999; 6: 343-5.
- Marshall VF, Marchetti AA, Krantz KE: The correction of stress incontinence by simple vesicourethral suspension. Surg Gynecol Obstet. 1941. 88: 509-18.
- Faerber GJ, Belville WD, Ohl DA, Plata A: Comparison of transurethral versus periurethral collagen injection in women with intrinsic sphincter deficiency. Tech Urol. 1998; 4: 124-7.
- Pannek J, Brands FH, Senge T: Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;166: 1350-3.
- Lightner D, Calvosa C, Andersen R, Klimberg I, Brito CG, Snyder J, et al.: A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double-blind study of Durasphere. Urol. 2001; 58: 12-5.
- Groutz A, Blaivas JG, Kesler SS, Weiss JP, Chaikin DC: Outcome results of transurethral collagen injection for female stress incontinence: assessment by urinary incontinence score. J Urol. 2000; 164: 2006-9.
- Elsergany R, Elgamasy AN, Ghoniem GM: Transurethral collagen injection for female stress incontinence. Int Gynecol J Pelvic Floor Dysfunct. 1998; 9: 13-8.
- Cross CA, English SF, Cespedes RD, McGuire EJ: A follow-up on transurethral collagen injection therapy for urinary incontinence. J Urol. 1998; 159: 106-8.
- Richardson TD, Kennelly MJ, Faerber GJ: Endoscopic injection of glutaraldehyde cross-linked collagen for the treatment of intrinsic sphincter deficiency in women. Urol. 1995; 46: 378-81.
- Clemens JQ, Bushman W: Urethral diverticulum following transurethral collagen injection. J Urol. 2001; 166: 626.
- Ginsberg DA, Boyd SD: Permanent urinary retention after transurethral injection of collagen. J Urol. 2002; 167 (2 Pt 1): 648.
- Stothers L, Goldenberg SL: Delayed hypersensitivity and systemic arthralgia following transurethral collagen injection for stress urinary incontinence. J Urol. 1998; 159: 1507-9.
- Sweat SD, Lighnter DJ: Complications of sterile abscess formation and pulmonary embolism following periurethral bulking agents. J Urol. 1999; 161: 93-6.
- Lee PE, Kung RC, Drutz HP: Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol. 2001; 165: 153-8.
- Santarosa RP, Blaivas JG: Periurethral injection of autologous fat for the treatment of sphincteric incontinence. J Urol. 1994; 151: 607-11.
- Haab F, Zimmern PE, Leach GE: Urinary stress incontinence due to intrinsic sphincter deficiency: experience with fat and collagen periurethral injections. J Urol. 1997; 157: 1283-6.
____________________
Received: April 17, 2002
Accepted: May 24, 2002
_______________________
Correspondence address:
Dr. Omid Rofeim
Department of Urology
Long Island Jewish Medical Center
270-05 76th Ave.
New Hyde Park, NY, 11021, USA
Fax: + 1 718 343-6254
E-mail: omidrofeim@hotmail.com