RESPONSIVENESS
TO THE PORTUGUESE VERSION OF THE INTERNATIONAL CONSULTATION ON INCONTINENCE
QUESTIONNAIRE - SHORT FORM (ICIQ-SF) AFTER STRESS URINARY INCONTINENCE
SURGERY
(
Download pdf )
JOSE T. N. TAMANINI, MIRIAM DAMBROS, CARLOS A. L. D’ANCONA, PAULO C. R. PALMA, N. RODRIGUES-NETTO JR
Center for Prevention and Treatment of Urogynecological Dysfunction, Jau, and Division of Urology, School of Medicine, University of Campinas, Campinas, Sao Paulo, Brazil
ABSTRACT
Objective:
To evaluate the reliability and responsiveness (internal and external)
of the Portuguese version of the ICIQ-SF. We assessed the responsiveness
of the ICIQ-SF after surgical procedures for the treatment of stress urinary
incontinence.
Materials and Methods: Prospective open
label study in 2 tertiary referral centers. Sixty-one patients of both
genders (54 female and 7 male) were enrolled. Patients were treated using
surgical procedures, mostly with synthetic sling (82%). Patients were
assessed before surgery and at least 1 month postoperatively using the
ICIQ-SF in its translated and validated Portuguese version. Patients also
underwent pre-operative urodynamic tests, Stamey incontinence grading
and pad usage assessments. After surgery, patients underwent stress tests,
Stamey incontinence grading and pad usage assessments.
Results: The mean age was 57.2 (±
11.6) years and the mean duration of follow-up was 7.2 months (±
4.5). Objective parameters such as urodynamic tests (by means of VLPP)
and pad usage had significant correlation with changes in post-treatment
scores on the ICIQ-SF (p = 0.0062 and p < 0.0001 respectively). The
responsiveness expressed in terms of standardized effect sizes (SES) and
standardized response means (SRM) was large for both questionnaires (p
< 0.0001).
Conclusion: The results showed high responsiveness
(large effect sizes I and II) for the Portuguese version of the ICIQ-SF,
indicating that this instrument is suitable for measuring outcomes in
clinical trials for Brazilian patients with stress urinary incontinence.
Key
words: urinary incontinence, stress; urodynamics; prostheses
and implants; quality of life; questionnaires
Int Braz J Urol. 2005; 31: 482-90
INTRODUCTION
Patient-reported
outcomes including symptoms, functional status and perceived quality of
life (QoL) are increasingly used alongside objective clinical measurements
to monitor the course of urinary incontinence (UI) and its treatment.
Treatment outcomes, as perceived and reported by patients, complement
clinical evidence and judgment of efficacy and effectiveness. A number
of measures have been developed to assess the perceived impact of UI,
particularly for women, who report symptoms consistent with UI prevalence
estimates of between 10% and 30% (1). The King’s Health Questionnaire
(KHQ) (2) was the first QoL questionnaire translated and validated into
Portuguese in Brazil (3). The International Consultation on Incontinence
Questionnaire-Short Form (ICIQ-SF) (4) is a brief instrument used to assess
the impact of UI in patients’ lives. It has been recently translated
into Portuguese and its psychometric parameters, such as validity and
reliability, have already been assessed (APPENDIX) (5). The usefulness
of instruments designed to measure changes within individuals over time
is dependent not only on their reliability and validity, but also on their
ability to detect minimal clinically important differences. This psychometric
property is called “responsiveness” (6). Responsiveness to
the KHQ in its Portuguese version has just been assessed (7). According
to Norman et al. (8), there has been an increasing emphasis on responsiveness
as an essential characteristic for the use of health-related quality of
life measures in studies of therapeutic interventions. The necessity for
distinguishing between responsiveness and validity remains because an
instrument can be valid and still fail to detect clinically important
changes when they occur (9).
Based on the simple construct that “the
goal of any therapy is to effect change in health status”, our aim
was to determine whether the Portuguese version of the ICIQ-SF can be
considered reliable before and after treatment, and whether it is sensitive
to change in continence status over time (internal and external responsiveness)
following surgery to treat genuine stress incontinence (GSI).
MATERIALS AND METHODS
Patients
A total of 61 consecutive patients of both
genders complaining of stress urinary incontinence that had been urodynamically
diagnosed before treatment. The patients were chosen from two tertiary
referral centers (seven male and nine female patients from the first tertiary
referral center; 45 female patients from the second tertiary referral
center) for this prospective open label study. Eligibility criteria included
patients aged over 18 years old who were undergoing surgery for stress
urinary incontinence with at least one month of follow up. Patients who
were pregnant or currently breastfeeding or who had clinically severe
cognitive dysfunction were not enrolled in the study.
Methods
Patients who underwent surgical procedures
to treat stress urinary incontinence between September/2003 and November/2004
in both centers were enrolled. They were assessed by subjective and objective
parameters, as well as by analysis of the QoL and incontinence impact
using the ICIQ-SF.
Measures
Subjective parameters: Stamey incontinence
grading (10) (0 = cured; 1 = leakage with stressful activities (coughing,
sneezing); 2 = leakage with minimally stressful activities (e.g. walking);
3 = leakage at all times, with any activity) and QoL impact on patients’
lives evaluated with the Portuguese version of the ICIQ-SF.
Objective parameters: urodynamic test Valsalva
leak point pressure (VLPP) (Uromaster, Dynamed/Brazil), according to the
standard protocol (11) and analysis of pad usage (yes/no). If pads were
used, the frequency of their usage was recorded (0 = no use; 1 = 1-2 units/day;
2 = 3-4 u/d and 3 = 4 u/d). If patient stated no leakage at all during
the postoperative period, no further urodynamic testing was performed.
Outcome
Standardization
Stress urinary incontinence was defined
as the involuntary leakage of urine during coughing, sneezing or physical
exertion. To assess objective outcomes such as stress test after surgical
procedure, all the patients were in standing position with the physiological
bladder filled to maximum vesical capacity (strong desire or sensation
to void without urge). Patients were asked to perform repeated coughing
and Valsalva maneuvers. Any transurethral urine leakage (even drops) was
recorded as an objective failure of the surgical procedure and the patients
underwent urodynamic testing. The patients were also asked to re-rate
their QoL assessment according to ICIQ-SF after at least one-month follow-up.
Quality
of Life Questionnaire
The International Consultation on Incontinence
Questionnaire - Short Form (ICIQ-SF) is a simple and brief self-administered
questionnaire used to assess the level and impact on QoL of urinary incontinence
specifically. It is comprised of 3 questions regarding frequency, severity
and QoL impact of the UI along with an eight-item scale, which assess
the possible causes or situations related to UI. The ICIQ-SF final score
is the result of the total of the scores from questions 3, 4 and 5.
Psychometric Properties Evaluation
Reliability
Cronbach’s alpha was calculated by
using pre- and post-treatment data to assess internal consistency, which
is considered the degree of association between the items and scale scores.
A minimum value of 0.70 for group comparison was desirable.
Responsiveness
Responsiveness refers to an instrument’s
ability to detect change (improvement or deterioration) that occurs as
a result of therapy or disease progression. Participants who have an indication
of clinical change, whether improvement or deterioration, should have
a parallel change in their scale scores, while participants who show no
change should have stable scale scores (12). Internal responsiveness is
the ability of a measurement to change over a particular pre-specified
time frame. External responsiveness reflects the extent to which changes
in a measurement over a specified time frame relate to corresponding changes
in a reference measurement of health status (13).
Statistical
Analysis
To assess ICIQ-SF reliability, standardized
Cronbach’s alpha was used. The assessment of internal responsiveness
involves statistical estimation of the size of the effect; i.e. an estimate
of the magnitude of the change in health status (13). Standardized effect
size (SES or ES I) and standardized response mean (SRM or ES II) provide
a standardized measurement of the change in score from an instrument.
Both SES and SRM can be considered large (> 0.80), moderate (0.5-0.8)
or small (< 0.5) (13). The Exact Mann-Whitney U - test for independent
groups was used to compare ICIQ-SF final scores to pad usage and VLPP
(external responsiveness). The McNemar test was used to assess significant
changes between proportions. Wilcoxon’s signed rank test was used
to compare the scores between follow-up and baseline. Statistical tests
were considered significant at the 5% level.
The software used was SAS (System for Windows
- Statistical Analysis System), version 8.2 (SAS Institute Inc., 1999-2001,
Cary, NC, USA). The statistical analysis was based on recommendations
by Husted et al. (13), Conover (14) and Fleiss (15).
Ethical
Approval
The study obtained prior approval from the
Ethical Committee of the Medical Sciences School, Unicamp (No. 261/2001).
All the participants signed the informed consent statement before being
included in this study.
RESULTS
The
socio-demographic characteristics of the ICIQ-SF sample population are
displayed in Table-1. Forty-six female (75.4%) and 4 male patients (6.6%)
underwent synthetic sling procedures; 7 female patients (11.5%) underwent
classic sling procedures; 1 female and 3 male patients (1.7% and 4.9%
respectively) underwent periurethral injection therapy. The mean Valsalva
leak point pressure (VLPP ± SD) was 80.5 ± 31.9 cm H2O.
The mean length of follow-up was 7.2 months (SD = ± 4.5).

The studies of ICIQ-SF reliability by means
of Cronbach’s alpha, before and after treatment, were 0.85 and 0.95
respectively.
The great majority of the patients (58 or
95.1%) had a negative stress test after surgical treatment for stress
urinary incontinence.
A total of 54 out of 61 patients (88.5%)
who filled out the ICIQ-SF considered themselves cured after surgical
treatment (p < 0.0001, Wilcoxon’s signed rank test).
Fifty (82%) out of 61 patients used pads
before surgical treatment. After the procedure only 4 (6.6%) were still
using them (p < 0.0001, McNemar test).
Twenty-five out of 61 patients (41%) changed
at least 3 pads per day before treatment. Nevertheless, 57 patients (93.5%)
did not use pads at all after surgery.
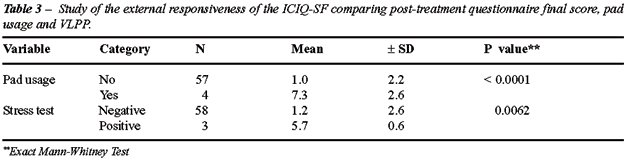
Responsiveness
Study
The study of the internal responsiveness
for ICIQ-SF is shown in Table-2. The internal responsiveness was quantified
using standardized effect size (SES or ES I) and standardized response
mean (SRM or ES II), which demonstrated a large effect size. Comparison
between pre- and post-operative scores of the ICIQ-SF is also shown in
Table-2. We found statistically significant differences between pre- and
post-treatment ICIQ-SF scores (p < 0.0001). Similar results were found
from the study of external responsiveness when the post-treatment final
score was compared with an external variable such as pad usage and stress
test (Table-3).

COMMENTS
This
study investigated the reliability and responsiveness of the ICIQ-SF in
Portuguese. Although it was not our main goal, we have now compared the
results from the ICIQ-SF responsiveness study with the results from the
just-published paper assessing the responsiveness of the Portuguese version
of the King’s Health Questionnaire.
Nowadays, Quality of Life questionnaires
are considered to be one of the most important outcome measures in many
clinical studies. Traditionally, there is a consensus that newly developed
instruments should be tested for validity and reliability before they
can be used in clinical trials. For evaluative instruments designed to
measure longitudinal changes in health-related quality of life over time,
responsiveness has been proposed as a third requirement (16).
The ICIQ-SF has just been validated and
translated into Portuguese (5). The King’s Health Questionnaire
differs from the ICIQ-SF in many aspects. KHQ was designed for female
patients only and assesses the impact of UI in eight domains of the patients’
lives. The more comprehensive instrument is, more detailed is the health
profile it provides (2). Contrarily, ICIQ-SF is brief and simple to understand
and fill in. Furthermore, it is not gender-specific like the KHQ and is
intended to be universal (applicable for use in individuals of all ages,
genders, backgrounds, patient groups, diagnoses and cultures). A further
disadvantage of the KHQ is that it does not attempt to determine clearly
the severity of incontinence in terms of the frequency or amount of leakage.
In addition, the KHQ has received limited use outside of clinical settings.
Conversely, the ICIQ-SF is intended to be brief yet comprehensive enough
for use in assessing incontinence and serves as an outcome measure in
clinical trials and other research to assess the efficacy of interventions.
Moreover, it is suitable for use as an epidemiological tool to determine
the distribution of incontinence in populations around the world, allowing
international multi-center studies to be made (4). One of the criticisms
of the QoL assessment by ICIQ-SF is that the QoL construct is based on
one question in a 10-item scale to quantify the burden of urinary incontinence
in the perceived quality of a patient’s life. It is to be used as
a visual analog scale (VAS) where the patients can choose the degree of
interference from UI in their lives by ticking or circling a number on
a scale ranging from zero to ten (when zero is considered “not interfere
at all” and 10 represents “interfere a lot”).
According to De Boer et al. (17), their
study assessing the role of VAS as a valid and reliable tool for the study
of QoL in clinical practice and research found that VAS could be considered
a good instrument when compared to multi-item questionnaires. Based on
their results, they recommend its use as a global quality of life measure
in clinical trials. Indeed, most QoL questionnaires are composed of many
items divided into several domains – such as role and physical limitation,
personal relationships, emotion, among others. The criticisms of these
questionnaires are that they are time-consuming and difficult to use outside
clinical settings. Contrarily, the brevity of the ICIQ-SF enables it to
be used without greatly increasing the burden on the respondents.
As shown in Table-1, 10 patients (16.4%)
were illiterate and an interviewer read the question to ensure important
data was not missed. According to data from the 2000 Census (18), the
illiteracy rate for Brazilian people of both sexes aged 15 or over is
13.3%, and the mean length of schooling was 5.7 years. Comparing the mean
level of schooling of our population sample with the literacy level for
the whole Brazilian population, it can be seen that our sample had almost
the same overall rate of literacy. These patients were considered unable
to understand the questions, so we chose to have the questionnaire read
out loud (without explanation of the questions) by a nursing professional
who was trained for this task. In our environment, such a procedure is
normal, especially in studies that utilize scales. It should be added
that, after comparing different methods of administering questionnaires,
Weinberger et al. (19) concluded that 70% of the patients preferred interviews
and 20% the self-administered forms.
Reliability as measured by Cronbach’s
Alpha showed a good to excellent degree of association between the scale
scores before and after treatment.
Internal responsiveness expressed in terms
of the SES and the SRM (Table-2) showed large values for effect size studies
for all ICIQ-SF scores. This study highlighted that ICIQ-SF was strongly
sensitive to change, enabling it to be used as an important tool in clinical
practice or research.
As shown in Table-3 (external responsiveness
study), there was a good correlation between the ICIQ-SF general score
after treatment regarding pad usage analysis. The ICIQ-SF general score
showed a significant difference when the two groups (yes/no) were compared
(p < 0.0001). Similar results were found when the ICIQ-SF scores were
also compared to the post-treatment analysis of Stress test (p = 0.0062),
showing excellent external responsiveness.
These results are in line to the responsiveness
study of KHQ, which yielded good results from the assessed parameters,
for both internal and external responsiveness, apart from having excellent
reliability (7).
Beaton et al. (20) proposed a new taxonomy
for responsiveness based not only on statistical characteristics such
as “large effect sizes” but also on a very highly contextualized
attribute of an instrument. There are three axes (questions) underlying
the new classification system: “who is being analyzed?” (individuals
or groups); “which scores are being contrasted?” (over time
/ one point in time); and “Which type of change is being quantified?”
(for example, observed change over important change). So, a questionnaire
could, thus, be described as being “responsive to” a given
category in this new taxonomy.
The present study demonstrates the relevance
of quality of life to the assessed clinical condition. The performance
of the ICIQ-SF in this clinical trial suggests that it can be an important
addition to the compendium of outcome measures used to assess UI and its
treatment. Both ICIQ-SF and KHQ are now available for use in clinical
practice and research in Brazil. Our next aim is to check ICIQ-SF sensitivity
in a large community-based population epidemiological study, as it is
also intended to be used.
In conclusion, the ICIQ-SF appears to capture
changes, i.e. it has good internal and external responsiveness. Although
brief, it seemed to be robust in the whole study of responsiveness, showing
large effect size and excellent correlation with clinical and surgical
outcome.
REFERENCES
- Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagner TH, Buesching DP: Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I-QOL). Urology. 1999; 53: 71-6. Erratum in: Urology 1999; 53: 1072.
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S: A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol. 1997; 104: 1374-9.
- Tamanini JT, D’Ancona CA, Botega NJ, Rodrigues Netto N Jr: Validation of the Portuguese version of the King’s Health Questionnaire for urinary incontinent women. Rev Saude Publica. 2003; 37: 203-11.
- Avery K, Donovan J, Abrams P: Validation of a new questionnaire for incontinence: the International Consultation on Incontinence Questionnaire (ICIQ). Abstract no. 86 of the International Continence Society 31st annual meeting. Seoul, Korea. Neurourol Urodyn. 2001; 20: 510-1.
- Tamanini JT, Dambros M, D’Ancona CA, Palma PC, Rodrigues Netto N Jr: Validation of the “International Consultation on Incontinence Questionnaire - Short Form” (ICIQ-SF) for Portuguese. Rev Saude Publica. 2004; 38: 438-44.
- Guyatt G, Walter S, Norman G: Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis. 1987; 40: 171-8.
- Tamanini JT, Dambros M, D’Ancona CA, Palma PC, Botega NJ, Rios LA, et al.: Concurrent validity, internal consistency and responsiveness of the Portuguese version of the King’s Health Questionnaire (KHQ) in women after stress urinary incontinence surgery. Int Braz J Urol. 2004; 30: 479-86.
- Norman GR, Stratford P, Regehr G: Methodological problems in the retrospective computation of responsiveness to change: the lesson of Cronbach. J Clin Epidemiol. 1997; 50: 869-79.
- Guyatt GH, Deyo RA, Charlson M, Levine MN, Mitchell A: Responsiveness and validity in health status measurement: a clarification. J Clin Epidemiol. 1989; 42: 403-8.
- Stamey TA: Urinary Incontinence in the Female. In: Campbell’s Urology, 4th ed., Harrison JH, Guittes RF, Perlmutter AD (eds.). Philadelphia, WB Saunders. 1979; pp. 2272-93.
- Nitti VW, Combs AJ: Correlation of Valsalva leak point pressure with subjective degree of stress urinary incontinence in women. J Urol. 1996; 155: 281-5.
- Lubeck DP, Prebil LA, Peeples P, Brown JS: A health related quality of life measure for use in patients wht urge urinary incontinence: A validation study. Qual Life Res. 1999; 8: 337-44.
- Husted JA, Cook RJ, Farewell VT, Gladman DD: Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000; 53: 459-68.
- Conover WJ: Practical Nonparametric Statistics. In: Contingency Tables and the Use of Ranks. New York. John Wiley & Sons. 1971; pp. 140-281.
- Fleiss, JL Statistical Methods for Rates and Proportions. 2nd ed. New York. John Wiley & Sons. 1981; pp 212-22.
- Terwee CB, Dekker FW, Wiersinga WM, Prummel MF, Bossuy PM: On assessing responsiveness of health-related quality of life instruments: guidelines for instrument evaluation. Qual Life Res. 2003; 12: 349-62.
- de Boer AG, van Lanschot JJ, Stalmeier PF, van Sandick JW, Hulscher JB, de Haes JC, et al.: Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004; 13: 311-20.
- IBGE Foundation (Brazilian Institute of Geography and Statistics). Minimal Social Indicators: Demographic Aspects - General Information. 1999. Available at http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/indicadoresminimos/tabela3.shtm [in Portuguese]
- Weinberger M, Oddone EZ, Sansa GP, Sandsman PB: Are health-related quality of life measures affected by the mode of administration? J Clin Epidemiol. 1996; 49: 135-40.
- Beaton DE, Bombardier C, Katz JN, Wright JG: A taxonomy for responsiveness. J Clin Epidemiol. 2001; 54: 1204-17.
_______________________
Received: February 2, 2005
Accepted after revision: May 3, 2005
_______________________
Correspondence
address:
Dr. Jose Tadeu Nunes Tamanini
Rua Floriano Peixoto, 443
Jau, SP, 17201-100, Brazil
Fax: + 55 14 3621-1829
E-mail: tadeutamanini@jau.flash.tv.br
In
order to incorporate in a single questionnaire the most relevant aspects
of urinary incontinence and its impact on quality of life, the ICIQ, which
is more widely used in research studies, was summarized in the ICIQ-SF
format, which is directed to clinical application in the daily practice.
The three questions on the ICIQ-SF consistently assess frequency, severity
and impact on quality of life caused by urinary incontinence. It can be
applied to patients of both genders, either young or elderly. Since 2001,
it has been validated in several other countries with a native language
different from English, such as Spain and Japan (1,2).
Its
recent validation for Portuguese was clearly important since it allows
the use of this assessment tool in studies on urinary incontinence conducted
in Brazil, thus enabling the comparison between results obtained here
with those published in urologic international literature.
The
present study, which was prospectively performed at two tertiary referral
centers, verified the responsiveness of ICIQ-SF, which was applied to
patients undergoing surgical treatment for urinary incontinence. The positive
correlation between urodynamic findings and e pad test and ICIQ-SF values,
as well as its responsiveness with a high level of statistical significance,
confirmed the applicability of this questionnaire in its Portuguese version.
Another
significant aspect regarding the ICIQ-SF questionnaire is the recent demonstration
(3) of equivalence in results – both when it is self-applied (according
to its initial proposal) and when it is completed by the physician. This
fact is important in our environment where a great deal of patients can
have difficulties in interpreting and completing the questionnaire.
REFERENCES
1. Espuna Pons M,
Rebollo Alvarez P, Puig Clota M: Validation of the Spanish version of
the International Consultation on Incontinence Questionnaire-Short Form.
A questionnaire for assessing the urinary incontinence. Med Clin (Barc).
2004; 122: 288-92.
2. Terai A, Ueda N, Utsunomiya N, Kouhei N, Ichioka K, Yoshimura K: Effect
of urinary incontinence on lower urinary tract symptoms in Japanese women.
Urology. 2004; 64: 1139-43.
3. Hajebrahimi S, Corcos J, Lemieux MC: International consultation on
incontinence questionnaire short form: comparison of physician versus
patient completion and immediate and delayed self-administration. Urology.
2004; 63: 1076-8.
Dr.José
Carlos Truzzi
Division of Urology
Santo Amaro University - UNISA
São Paulo, SP, Brasil
E-mail: jctruzzi@hotmail.com