PROSTATIC
SPECIFIC ANTIGEN FOR PROSTATE CANCER DETECTION
( Download pdf )
LUCAS NOGUEIRA, RENATO CORRADI, JAMES A. EASTHAM
Department of Surgery (LN, JAE), Memorial Sloan-Kettering Cancer Center, New York, NY, USA and School of Medicine (RC), Belo Horizonte, Minas Gerais, Brazil
ABSTRACT
Prostate-specific antigen (PSA) has been used for prostate cancer detection since 1994. PSA testing has revolutionized our ability to diagnose, treat, and follow-up patients. In the last two decades, PSA screening has led to a substantial increase in the incidence of prostate cancer (PC). This increased detection caused the incidence of advanced-stage disease to decrease at a dramatic rate, and most newly diagnosed PC today are localized tumors with a high probability of cure. PSA screening is associated with a 75% reduction in the proportion of men who now present with metastatic disease and a 32.5% reduction in the age-adjusted prostate cancer mortality rate through 2003. Although PSA is not a perfect marker, PSA testing has limited specificity for prostate cancer detection, and its appropriate clinical application remains a topic of debate. Due to its widespread use and increased over-detection, the result has been the occurrence of over-treatment of indolent cancers. Accordingly, several variations as regards PSA measurement have emerged as useful adjuncts for prostate cancer screening. These procedures take into consideration additional factors, such as the proportion of different PSA isoforms (free PSA, complexed PSA, pro-PSA and B PSA), the prostate volume (PSA density), and the rate of change in PSA levels over time (PSA velocity or PSA doubling time). The history and evidence underlying each of these parameters are reviewed in the following article.
Key
words: prostate cancer; diagnosis; prostate-specific antigen;
biopsy
Int Braz J Urol. 2009; 35: 521-31
INTRODUCTION
Prostate-specific
antigen (PSA) was approved by the United States Food and Drug Administration
(FDA) in 1986 to monitor men with prostate cancer (PC). In 1994, it was
approved for cancer detection. PSA testing revolutionized our ability
to diagnose, treat, and follow-up patients. In the last decades, PSA screening
has led to a substantial increase in the incidence of PC. This increased
detection has caused the incidence of advanced-stage disease to decrease
at a dramatic rate, and most recently diagnosed PC today are localized
tumors with a high probability of cure (1).
Despite the shift toward improved detection
and early diagnosis, controversy still exists regarding the merits of
screening. As a result of PSA screening, the lifetime risk of being diagnosed
with PC has increased to 16%, whereas the risk of dying from the disease
is only 3.4% (2). Increased detection of slow-growing or relatively benign
cancer can be a contributing factor to the large discrepancy between incidence
and mortality rates. These cancers do not necessarily require definitive
treatment, raising concerns about overdiagnosis and overtreatment. Patients
with non-life-threatening disease may unnecessarily be exposed to sexual,
urinary, and bowel dysfunction that can occur after any therapy for PC
(3).
There is currently no consensus among health organizations regarding routine
PSA screening for PC. Opponents claim there is no conclusive evidence
that early detection and treatment influence the overall death rate, and
screening can result in great morbidity. However, there is evidence that
screening is responsible for a decrease in cancer-specific mortality.
Bartsch et al. assessed PC mortality in Tyrol, Austria. In this region,
86.6% of men had gone through PSA testing at least once, and radical prostatectomy
was the primary treatment option. Cancer mortality declined at a significantly
faster rate in Tyrol than in the rest of Austria, where screening was
not as widely used (54% vs. 19%, P = 0.001). The investigators concluded
that the reduction in mortality was probably due to early detection, consequent
down-staging and effective treatment (4).
The Prostate, Lung, Colorectal, and Ovarian
Cancer Screening Trial and the European Randomized Study of Screening
for Prostate Cancer are two large, randomized studies addressing the question:
does screening improve prostate cancer-specific mortality? It is hoped
that both studies can provide further insight into PSA testing and its
role in reducing prostate cancer mortality.
PSA is a valuable tool for detecting PC,
but it is not perfect. The test lacks both the sensitivity and specificity
to accurately detect the presence of PC. PSA is a prostate-specific marker,
not a PC marker. Elevated levels in the blood may be driven by conditions
such as benign prostatic hyperplasia (BPH) and prostatitis (5). None of
the PSA cut-offs currently in use consistently identify patients with
PC and exclude patients without cancer. PC incidence in patients with
PSA levels below the accepted level of 4.0 ng/mL is similar to the incidence
of prostate cancer in patients with PSA between 4.0-10.0 ng/mL, which
leads some experts to state that it should not be used as a PC marker
(6).
The issues regarding PSA accuracy have led investigators to evaluate additional
methods of analyzing PSA data, including the use of PSA derivatives and
others biomarkers to improve PSA efficacy in detecting PC. Despite the
discovery of many new biomarkers, only a few have shown some clinical
value.
PSA BIOLOGY
PSA
is a serine protease member of the human kallikrein family. It is produced
in both normal and cancerous prostate tissue and secreted into seminal
fluid. Its physiologic function is to liquefy semen from its gel form
(7). Normal prostate architecture keeps PSA confined to the gland, and
only a small portion is leaked into the circulation. PSA circulates in
free and complexed forms. Free forms represent 5%-35% of total PSA. Complexed
forms (65%-95%) are bound to protease inhibitors. Binding inactive protease
and PSA in the blood has no catalytic activity (8).
Serum PSA elevations occur as a result
of disruptions in the prostate architecture that allow PSA to enter the
circulation. This can occur in disease settings (PC, BPH, or prostatitis)
or after prostate manipulation (massage, biopsy, or transurethral resection).
Increased levels in PC patients cannot be explained by increased synthesis.
In fact, PSA expression is slightly decreased in cancer tissue (9).
PSA expression is strongly influenced by
androgens. Patients using 5a-reductase agents such finasteride and dutasteride
show a 50% decrease in detectable PSA level and should have their level
doubled to reflect the correct estimated PSA level (10).
Ethnicity, age, and body mass index (BMI)
can also influence PSA levels. Black men without PC show higher levels
compared with white men, probably reflecting a higher expression by benign
prostate tissue (11). Lower levels of PSA in obese men, which may be related
to the influence of estrogen, can mask the presence of significant cancer
(12).
PSA AS A DETECTION TOOL FOR PROSTATE CANCER
Prostate
cancer risk varies according to serum PSA levels. Initially, a threshold
of 4.0 ng/mL was recommended as the level at which a man should undergo
prostate biopsy. This value was based on studies on healthy men showing
that 97% of men older than 40 had PSA levels = 4.0 ng/mL. Sensitivity
and specificity of this threshold were 20% and 94%, respectively (13).
Moreover, the 4.0 ng/mL threshold has a positive predictive value of only
37% and a negative predictive value of 91%, which translates into a 25%
probability that a man in the 4.0-10.0 ng/mL zone has cancer (14).
Since prostate biopsies are rarely performed
on men with low PSA levels, specificity and sensitivity of PSA are more
difficult to validate. The Prostate Cancer Prevention Trial was the first
study to assess PC incidence and aggressiveness in men with low PSA levels
and a normal digital rectal examination. The trial was designed to examine
the association between finasteride and PC risk; prostate biopsies were
offered to all men in the placebo arm at the end of the 7-year study.
Overall, cancer detection in the placebo group was 15%, and high-grade
prostate cancer was found in 15% of the patients. Among men with PSA levels
= 0.5, 0.6-1.0, 1.1-2.0, 2.1-3.0, and 3.1-4.0 ng/mL, the incidence of
prostate cancer was 7%, 10%, 17%, 24%, and 27%, respectively. The cancer
incidence in patients with PSA levels above 2.0 ng/mL differed only slightly
from those with PSA between 4.0 and 10.0 ng/mL (15). This study revealed
that PC is not rare with a PSA below 4.0 ng/mL, and aggressive PC was
found even in patients with PSA levels below 1.0 ng/mL.
Table-1 shows the sensitivity and specificity
of different PSA thresholds. Attempts to improve detection by lowering
the PSA threshold are subject to a higher false positive rate. For example,
lowering the threshold to 2.6 ng/mL would raise sensitivity to 40%, but
it would increase the false positive rate to 18.9%, translating into more
unnecessary biopsies. Thresholds higher than 4.0 ng/mL would miss some
aggressive diseases. Catalona et al. demonstrated that one third of prostate
cancers detected with PSA above 4.0 ng/mL already had extracapsular disease,
and the likelihood of having organ-confined disease at radical prostatectomy
was 81%, 74%, and 72% in men with PSA levels of 2.6-4.0, 4.1-7.0, and
7.1-10.0, respectively (16).

Positive PC familiar history is also important.
In those men, the likelihood of PC diagnosis in is 20%, 13%, 17.9%, 29.4%
and 77.8% in men with PSA levels of < 0.5, 0.5-1.0, 1.1-2.0, 2.1-3.0,
and 3.1-4.0, respectively (17).
The threshold of 4.0 ng/mL has been criticized
both for not being able to identify cancer (including high-grade) in patients
and for encouraging unnecessary prostate biopsies. Establishing a single
PSA cutoff for recommending biopsy might be inappropriate. No single value
can definitively place men into groups of high and low risk (18). PSA
is not diagnostic; it helps assess each man’s risk for PC and should
be used together with other parameters to decide when a prostate biopsy
would be appropriate.
PSA DERIVATIVES
PSA derivatives represent permutations of total PSA that have been tested in clinical practice to improve its sensitivity and specificity. These methods can help identify patients at risk for PC when total PSA (tPSA) does not clearly identify them. The use of PSA derivatives provides a better understanding of an individual’s risk, allowing improved detection rates while avoiding unnecessary biopsies.
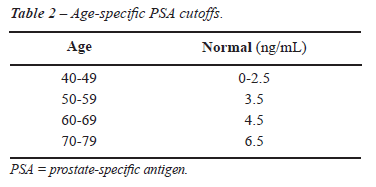
PSA AND AGE
PSA
levels vary through life, but the median PSA level increases over time,
mainly after the age of 50, when prostate conditions such as benign prostatic
hyperplasia (BPH), prostatitis, and PC become more common. Age-specific
PSA reference ranges have been proposed as a means of increasing sensitivity
of detection in younger men and specificity in older men. Different thresholds
have been established based on the 95th percentile among healthy populations.
It was hoped that matching the PSA threshold to the patient’s age
would avoid unnecessary biopsies and overdetection in older men while
diagnosing more instances of cancer in younger men (Table-2). However,
further studies, showed that age-specific PSA cutoffs missed 20% to 60%
of cancer in men older than 60 years of age (19). Because of this lack
of sensitivity, age-specific PSA has not been uniformly accepted.
Studies have indicated that PSA level increases even decades before PC
has been diagnosed. Loeb et al. studied 1,178 men in their 40s with risk
factors for PC. The risk of subsequent cancer detection was 14.6-fold
higher for men with a baseline PSA level between 0.7 and 2.5 ng/mL than
for men with levels of < 0.7 ng/mL (20). In a cardiovascular risk assessment
study of 21,227 men in Sweden, Lilja et al. showed increased PSA levels
up to 20 years before clinical manifestation of advanced disease. Men
with PSA 1.01-2.0 ng/mL had a 2.5-fold increased risk of PC compared to
men with PSA = 0.5 ng/mL, corresponding to a long-term risk close to the
population mean. PSA levels between 2.01-3.0 ng/mL were associated with
a 19-fold increased risk of cancer. There was also an increased risk of
advanced PC. PSA screening was not widely used in Sweden at the time of
this study, thus this population could be used to demonstrate the natural
evolution of PC without the interference of PSA screening. The authors
suggested that an early PSA test should be done, not for detection of
cancer, but to stratify the cancer risk, and for subsequent intervention.
Men with PSA > 2.0 ng/mL should be closely followed, while those with
PSA below this level should undergo a less frequent follow-up. Such strategy
may largely eliminate the poor sensitivity associated with BPH (21).
PSA DENSITY
Although
PSA expression is higher in men with BPH, prostate cancer tissue releases
more PSA into circulation (22). Volume-based prostate parameters have
been evaluated to better interpret PSA levels in men with large prostates.
Patients with BPH have transition zone
(TZ) enlargement; most prostate cancers arise in the peripheral zone (PZ).
Adjusting PSA to account for TZ volume has been evaluated as a method
of distinguishing PC from BPH. Thresholds of 0.23 and 0.38 ng/mL/cm3 were
proposed for TZ volumes above 20 cc and below 20 cc, respectively (23).
PSA density (PSAD) is the serum PSA level divided by prostate volume as
assessed by transrectal ultrasound. A direct relationship between PSAD
and the risk of cancer was reported by Seaman et al. (24). PSAD cutpoints
between 0.10 and 0.18 ng/mL/cc were proposed as the levels that should
prompt prostate biopsy. However, using 0.15 ng/mL/cc as the cutoff, Catalona
et al. found that half of the cancers detected in men with PSA between
4.0 and 10.0 ng/mL would have been missed. Lower cutpoints appear to maximize
sensitivity and specificity. PSAD has also been associated with tumor
aggressiveness and treatment outcomes (25).
PSAD is not widely used, as it is an uncomfortable,
invasive method requiring skillful performance of transrectal ultra-sonography
in which accuracy is influenced by the shape of the prostate. Furthermore,
it is more time consuming and expensive than a simple blood test.
PSA VELOCITY AND PSA DOUBLING TIME
The
rate at which PSA levels change can help distinguish between patients
with BPH and PC. Carter et al. first described this concept, known as
PSA velocity (PSAV), in 1992 (26). They measured PSA levels in frozen
sera taken from 54 men already participating in a longitudinal study on
aging. Long-term serial PSA measurements showed that the men who eventually
developed prostate cancer experienced a marked difference in the rate
of change years before their diagnosis. PSAV greater than 0.75 ng/mL per
year was significantly associated with PC. This cutpoint has shown a 79%
sensitivity and 90% specificity in detecting prostate cancer in men with
PSA levels between 4.0 and 10.0 ng/mL. Sensitivity, however, dropped to
11% in patients with PSA below 4.0 ng/mL. More recently, cutoffs of 0.1-0.5
ng/mL per year were proposed to recommend prostate biopsy for men within
this PSA range (27). As high PSAV is rare when PSA levels are low, further
studies are needed to evaluate PSAV cutoff with low PSA levels.
The clinical use of PSAV is limited. Physiological
fluctuations in PSA levels and differences in assay standardization compromise
its use in the short term. Moreover, recent reports have questioned its
role in PC detection. There is little evidence showing that calculation
of PSA velocity in untreated patients provides predictive information
beyond that provided by absolute PSA level alone (28,29).
PSA doubling time (PSADT) is the time required
for PSA to double. It is mostly used to monitor disease progression after
radical therapy or as a parameter to decide when patients treated with
active surveillance should undergo radical therapy. PSADT has not been
shown to be useful in prostate cancer diagnosis.
PSA ISOFORMS
Free PSA and Complexed PSA
PSA
exists in several forms. The majority binds to protease inhibitors (mostly
ACT) and is known as complexed PSA (cPSA). Approximately 5%-35% of tPSA
is not bound and is known as free PSA (fPSA) (30). Current immunoassays
can detect both cPSA and fPSA forms in the serum.
The ratio of fPSA to tPSA has been used
to increase specificity for PC and to reduce unnecessary biopsies. The
proportion of PSA that is complexed to ACT (cPSA) is higher and the percentage
of fPSA is correspondingly lower in patients with prostate cancer (31).
Thus, the percentage ratio of fPSA (%fPSA) over tPSA is greater in men
without PC, and provides additional specificity in detection, as shown
by Catalona et al. They reported that in men with tPSA between 4.0 and
10.0 mg/mL, cancer incidence was only 8% if the %fPSA was > 25%; whereas
56% of men were found to have cancer if the %fPSA was less than 10% (Table-3).
They also reported that %fPSA > 15% was related to favorable pathological
features at radical prostatectomy. They proposed a %fPSA cutoff of 25%
as the level at which a prostate biopsy is indicated (32). Further studies
also demonstrate the utility of %fPSA. Values varying from 14% to 28%
were proposed, which would avoid 20% to 65% of unnecessary prostate biopsies.

The use of %fPSA in men with PSA levels
below 4.0 ng/mL remains controversial. In a prospective study of 883 men
with PSA levels between 2.0 and 3.9 ng/mL, Raaijmakers et al. showed that
only 9% of unnecessary biopsies would be avoided. However, more recent
data demonstrated the utility of %fPSA in patients with this PSA range
(33). In a group of patients with PSA < 4.0 ng/mL, Djavan et al. showed
that a %fPSA cutoff of 27% had a sensitivity of 90% and prevented 18%
of unnecessary biopsies (34).
Prostate volume has been shown to influence
the %fPSA ratio. Larger prostate in patients with PC is correlated with
higher %fPSA (35), detection rates are higher in patients with small prostates.
Therefore, different %fPSA were proposed in order to avoid unnecessary
biopsies. To maintain 90% sensitivity, a %fPSA of 23% and 14% should be
used to indicate a prostate biopsy in prostate of less than or more than
40 cc, respectively (36). This strategy would compensate for the dilution
effect caused by large prostates. In men receiving 5a-reductase agents,
both tPSA and fPSA levels are decreased and the %fPSA is not altered.
Complexed PSA forms are bound with protease
inhibitors, and cPSA serum levels can be determined either by specific
assays or by subtracting fPSA from tPSA. Men with prostate cancer have
higher levels of cPSA than men without cancer; thus improved specificity
of tPSA is suggested. Regarding its clinical use, the 3.2 ng/mL cutoff
was estimated to be equivalent to the 4.0 ng/mL tPSA threshold and shows
similar diagnostic performance in cancer detection (37). To date, no study
has shown superiority of the cPSA or the cPSA/tPSA ratio, compared with
%fPSA, to enhance the specificity of prostate cancer detection (38). Despite
the fact that, theoretically, cPSA has a small advantage compared to tPSA
as a first-line parameter, only the ratio of cPSA to tPSA could reach
specificity levels comparable to %fPSA.
Free PSA Isoforms
Free
PSA exists as three distinct forms: proPSA, benign PSA (BPSA), and intact
PSA (iPSA). ProPSA is an inactive form and is found in increased proportions
in patients with PC (39). Mikolajczyk et al. found an overrepresentation
of the proPSA (-2) form in serum samples from PC patients compared with
BPH samples (40). Catalona et al. showed that use of percentage of proPSA
(proPSA/fPSA x 100) improved the specificity of PC detection and decreased
the number of unnecessary biopsies in men with tPSA between 2.0 ng/mL
and 4.0 ng/mL (41). Others studies have demonstrated that proPSA has enhanced
specificity over the use of PSA and %fPSA. However, a recent trial with
2,055 men showed no improvement in accuracy when these forms were compared
(42).
BPSA is formed when iPSA is cleaved at
amino-acid residues Lys145-146 and Lys182-183, and is present in prostate
tissue, serum, and seminal fluid. BPSA has been associated with prostate
volume and is highly associated with the transition zone; its levels are
increased in patients with BPH. BPSA levels have shown potential for improved
distinction of PC from BPH (33). Although it was shown that BPSA represents
0%-60% of fPSA, this measure does not have any clinical use, due to low
levels of BPSA in the blood. However, BPSA can be a marker for BPH and
may enhance specificity of %fPSA in combination with pro forms of fPSA,
or it may be used for therapeutic control of BPH.
Intact PSA is an uncleaved form of PSA, and it is similar to native PSA
except it is enzymatically inactive. There are no differences in iPSA
levels in men with or without cancer, but the ratio of this marker to
fPSA was significantly higher in men with cancer (43). Additional research
is under way to determine whether iPSA can improve the accuracy of PC
detection, as well as determine if iPSA levels are related to cancer aggressiveness
and treatment outcomes.
Even with the development of new assays
and discovery of PSA isoforms, there is still no agreement on how best
to improve the detection rate in clinical practice. Using a single PSA
cutoff is inappropriate because it misses a significant number of cases.
Any attempt to improve the detection rate will be subject to a lower specificity.
The use of PSA derivatives is not widespread, mainly because among them,
only %fPSA ratio alone has proved to be an addition. This scenario indicates
the need for a new biomarker that can improve specificity of prostate
cancer detection without poor sensitivity.
CONCLUSION
Although
PSA is one of the most valuable cancer markers, it is far from perfect.
PSA screening can lead to unnecessary biopsies, and overdiagnosis and
overtreatment of clinically insignificant prostate cancer. Standard thresholds
miss some clinically significant cancer, and no cutoff has reached high
sensitivity while preserving an acceptable specificity. Instead of having
a static threshold, PSA levels should be considered as an indicator of
risk to be weighted in combination with PSA derivatives, PSA isoforms,
and clinical features such as age, ethnicity, and BMI. The combination
of these data can be analyzed through multivariate logistic regression
models, nomograms, or artificial neural networks, which would calculate
each man’s risk of having PC. Those with higher risk would undergo
prostate biopsy.
Early baseline PSA measurement could be
useful in identifying men who are at a higher risk of developing PC in
the future, and they can be directed to a more intensive surveillance
protocol than men with low risk for cancer. Men with higher levels should
be closely followed, while those with normal levels should undergo a less
frequent follow-up. This strategy would facilitate a higher rate of diagnosis
in younger men while avoiding overdiagnosis in older men.
New markers have been discovered with the
help of recent technology, and future studies will demonstrate their usefulness
in PC detection. Until then, PSA will remain as the cornerstone of PC
screening and detection.
ACKNOWLEDGEMENTS
This study was supported by The Sidney Kimmel Center for Prostate and Urologic Cancers.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Ung JO, Richie JP, Chen MH, Renshaw AA, D’Amico AV: Evolution of the presentation and pathologic and biochemical outcomes after radical prostatectomy for patients with clinically localized prostate cancer diagnosed during the PSA era. Urology. 2002; 60: 458-63.
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK: SEER Cancer Statistics Review, 1975-2004, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site, 2007.
- Smither AR, Guralnick ML, Davis NB, See WA: Quantifying the natural history of post-radical prostatectomy incontinence using objective pad test data. BMC Urol. 2007; 7: 2.
- Bartsch G, Horninger W, Klocker H, Pelzer A, Bektic J, Oberaigner W, et al.: Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int. 2008; 101: 809-16.
- Klein LT, Lowe FC: The effects of prostatic manipulation on prostate-specific antigen levels. Urol Clin North Am. 1997; 24: 293-7.
- Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J: The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004; 172: 1297-301.
- Lilja H: A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985; 76: 1899-903.
- Piironen T, Nurmi M, Irjala K, Heinonen O, Lilja H, Lövgren T, et al.: Measurement of circulating forms of prostate-specific antigen in whole blood immediately after venipuncture: implications for point-of-care testing. Clin Chem. 2001; 47: 703-11.
- Qiu SD, Young CY, Bilhartz DL, Prescott JL, Farrow GM, He WW, et al.: In situ hybridization of prostate-specific antigen mRNA in human prostate. J Urol. 1990; 144: 1550-6.
- Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG: The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006; 176: 868-74.
- Fowler JE Jr, Bigler SA, Kilambi NK, Land SA: Relationships between prostate-specific antigen and prostate volume in black and white men with benign prostate biopsies. Urology. 1999; 53: 1175-8.
- Baillargeon J, Pollock BH, Kristal AR, Bradshaw P, Hernandez J, Basler J, et al.: The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005; 103: 1092-5.
- Myrtle J, Ivor L: Measurement of PSA in Serum by two Iimmunometric Methods (Hybritech Tandem-R/Tandem-E PSA). In: Catalona WJ (ed.), Clinical Aspects of Prostate Cancer. New York, Elsevier. 1989, pp. 161-71.
- Bunting PS: Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta. 2002; 315: 71-97.
- Thompson IM, Chi C, Ankerst DP, Goodman PJ, Tangen CM, Lippman SM, et al.: Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst. 2006; 98: 1128-33.
- Antenor JA, Roehl KA, Eggener SE, Kundu SD, Han M, Catalona WJ: Preoperative PSA and progression-free survival after radical prostatectomy for Stage T1c disease. Urology. 2005; 66: 156-60.
- Canby-Hagino E, Hernandez J, Brand TC, Troyer DA, Higgins B, Ankerst DP, et al.: Prostate cancer risk with positive family history, normal prostate examination findings, and PSA less than 4.0 ng/mL. Urology. 2007; 70: 748-52.
- Ulmert D, Serio AM, O’Brien MF, Becker C, Eastham JA, Scardino PT, et al.: Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008; 26: 835-41.
- Borer JG, Sherman J, Solomon MC, Plawker MW, Macchia RJ: Age specific prostate specific antigen reference ranges: population specific. J Urol. 1998; 159: 444-8.
- Loeb S, Roehl KA, Antenor JA, Catalona WJ, Suarez BK, Nadler RB: Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology. 2006; 67: 316-20.
- Lilja H, Ulmert D, Björk T, Becker C, Serio AM, Nilsson JA, et al.: Long-term prediction of prostate cancer up to 25 years before diagnosis of prostate cancer using prostate kallikreins measured at age 44 to 50 years. J Clin Oncol. 2007; 25: 431-6.
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E: Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987; 317: 909-16.
- Djavan B, Zlotta AR, Byttebier G, Shariat S, Omar M, Schulman CC, et al.: Prostate specific antigen density of the transition zone for early detection of prostate cancer. J Urol. 1998; 160: 411-8; discussion 418-9.
- Seaman EK, Whang IS, Cooner W, Olsson CA, Benson MC: Predictive value of prostate-specific antigen density for the presence of micrometastatic carcinoma of the prostate. Urology. 1994; 43: 645-8.
- Catalona WJ, Richie JP, deKernion JB, Ahmann FR, Ratliff TL, Dalkin BL, et al.: Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994; 152: 2031-6.
- Carter HB, Morrell CH, Pearson JD, Brant LJ, Plato CC, Metter EJ, et al.: Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res. 1992; 52: 3323-8.
- Fang J, Metter EJ, Landis P, Chan DW, Morrell CH, Carter HB: Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001; 58: 411-6.
- Vickers AJ, Savage C, O’Brien MF, Lilja H: Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009; 27: 398-403.
- Etzioni RD, Ankerst DP, Weiss NS, Inoue LY, Thompson IM: Is prostate-specific antigen velocity useful in early detection of prostate cancer? A critical appraisal of the evidence. J Natl Cancer Inst. 2007; 99: 1510-5.
- Christensson A, Björk T, Nilsson O, Dahlén U, Matikainen MT, Cockett AT, et al.: Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993; 150: 100-5.
- Lilja H, Christensson A, Dahlén U, Matikainen MT, Nilsson O, Pettersson K, et al.: Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991; 37: 1618-25.
- Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al.: Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998; 279: 1542-7.
- Raaijmakers R, Blijenberg BG, Finlay JA, Rittenhouse HG, Wildhagen MF, Roobol MJ, et al.: Prostate cancer detection in the prostate specific antigen range of 2.0 to 3.9 ng/ml: value of percent free prostate specific antigen on tumor detection and tumor aggressiveness. J Urol. 2004; 171: 2245-9.
- Djavan B, Zlotta A, Remzi M, Ghawidel K, Basharkhah A, Schulman CC, et al.: Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol. 2000; 163: 1144-8; discussion 1148-9.
- Partin AW, Catalona WJ, Southwick PC, Subong EN, Gasior GH, Chan DW: Analysis of percent free prostate-specific antigen (PSA) for prostate cancer detection: influence of total PSA, prostate volume, and age. Urology. 1996; 48(6A Suppl): 55-61.
- Catalona WJ, Smith DS, Wolfert RL, Wang TJ, Rittenhouse HG, Ratliff TL, et al.: Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995; 274: 1214-20.
- Allard WJ, Zhou Z, Yeung KK: Novel immunoassay for the measurement of complexed prostate-specific antigen in serum. Clin Chem. 1998; 44: 1216-23.
- Partin AW, Brawer MK, Bartsch G, Horninger W, Taneja SS, Lepor H, et al.: Complexed prostate specific antigen improves specificity for prostate cancer detection: results of a prospective multicenter clinical trial. J Urol. 2003; 170: 1787-91.
- Steuber T, O’Brien MF, Lilja H: Serum markers for prostate cancer: a rational approach to the literature. Eur Urol. 2008; 54: 31-40.
- Mikolajczyk SD, Marker KM, Millar LS, Kumar A, Saedi MS, Payne JK, et al.: A truncated precursor form of prostate-specific antigen is a more specific serum marker of prostate cancer. Cancer Res. 2001; 61: 6958-63.
- Catalona WJ, Bartsch G, Rittenhouse HG, Evans CL, Linton HJ, Amirkhan A, et al.: Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml. J Urol. 2003; 170: 2181-5.
- Lein M, Semjonow A, Graefen M, Kwiatkowski M, Abramjuk C, Stephan C, et al.: A multicenter clinical trial on the use of (-5, -7) pro prostate specific antigen. J Urol. 2005; 174: 2150-3.
- Nurmikko P, Pettersson K, Piironen T, Hugosson J, Lilja H: Discrimination of prostate cancer from benign disease by plasma measurement of intact, free prostate-specific antigen lacking an internal cleavage site at Lys145-Lys146. Clin Chem. 2001; 47: 1415-23.
____________________
Accepted after revision:
March 20, 2009
_______________________
Correspondence address:
Dr. Lucas Nogueira
Section of Urology, Department of Surgery
Memorial Sloan-Kettering Cancer Center
1275 York Avenue
New York, NY, 10065, USA
Fax: + 1 212 988-0760
E-mail: nogueirl@mskcc.org
EDITORIAL COMMENT
In this review article by Nogueira et al., the authors discuss the diagnostic
and therapeutic utility of using serum prostate specific antigen (PSA)
in the screening for prostate cancer. The authors highlight many of the
merits and drawbacks of using serum PSA as a screening tool. As serum
PSA cutoffs prompting prostatic biopsies are lowered, the incidence of
a negative prostatic biopsy increases (i.e. the positive predictive value
of the biopsy decreases). As well, this results in the overdetection of
clinically insignificant prostate cancer which has a low associated risk
of local-regional and systemic progression without active treatment. This
leads to the ultimate question: How can we identify the patients with
prostate cancer at high-risk of progression and then select these patients
for risk-appropriate treatment modalities?
In evaluating the utility of a screening
tool, several criteria that should be met are: 1) Does the screening test
detect the disease before it becomes clinically detectable? 2) Is the
test non-invasive and easy to perform in a clinic setting? 3) Does the
earlier detection of the disease using this screening tool alter the natural
history of the disease? 4) Is the test cost effective? For the most part,
PSA meets several of these criteria (namely criteria 1, 2, and 4) however
the major hindrance with the test is that although we have clearly noted
a stage migration in prostate cancer since integration of serum PSA as
a screening tool, it remains unclear and still debated whether there is
a survival benefit in screening a patient population with serum PSA. Two
large prospective clinical trials were recently published: The Prostate,
Lung, Colorectal, and Ovarian Cancer Screening (PLCO) Trial by Andriole
et al. (1) and the European Randomized Study of Screening for Prostate
Cancer (ERSPC) by Schröder et al. (2). These studies have attempted
to address this question in U.S. and European patient cohorts, respectively.
Although the study design and clinical criteria differed among these two
studies, they had very contrasting conclusions in that the PLCO study
concluded after 7 to 10 years of follow-up, the death rate of prostate
cancer in a PSA screening population did not differ from that in a non-screened
patient population whereas in the ERSPC trial, the authors concluded that
PSA screening resulted in a reduction in prostate cancer related deaths
by 20% but was associated with a high-risk of overdiagnosis. Although
the reasons for these significantly differing conclusions in both studies
will be argued for many years to come, it remains that at this point in
time, we still cannot convincingly demonstrate that there is a clear and
definitive survival benefit to using serum PSA as a screening tool. Furthermore,
many of our governing bodies and associations will not take a firm position
on supporting the role of serum PSA as a screening tool until this survival
benefit is convincingly demonstrated.
In those patients currently seeking prostate
cancer screening, a combination of digital rectal examination and serum
PSA screening currently remains the standard to which other screening
tools must be compared. In addition, an evolving view among many urologists
and oncologists is that a baseline serum PSA should be obtained in most
male patients at the age of 40 years old to help stratify those patients
at increased risk of prostate cancer and those best suited for rigorous
screening. Similarly, recent reports would suggest that a rapid rise in
the serum PSA (greater than 2 ng/mL rise) in the year prior to the diagnosis
of prostate cancer helps further define those patients at increased risk
of disease-progression and for whom, a high-risk treatment protocol may
best be suited.
There are clear limitations to using serum
PSA as a screening tool for prostate cancer and new novel tissue, serological,
and urinary markers will likely replace PSA in the not too distant future.
However, at this time, serum PSA remains the most utilized and useful
screening tool in our diagnostic armamentarium. The onus now lies on the
scientific community to develop and validate a better screening tool which
can identify those patients at increased risk of disease progression and
for whom definitive local or multimodal therapy is best suited.
REFERENCES
- Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al.: Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009; 360: 1310-9. Erratum in: N Engl J Med. 2009; 360: 1797.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al.: Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009; 360: 1320-8.
Dr. Philippe E. Spiess
Division of Urology
H. Lee Moffitt Cancer Center
Tampa, Florida, USA
E-mail: philippe.spiess@moffitt.org