EFFICACY
OF TRANSCUTANEOUS FUNCTIONAL ELECTRICAL STIMULATION ON URINARY INCONTINENCE
IN MYELOMENINGOCELE: RESULTS OF A PILOT STUDY
(
Download pdf )
Neurourology
doi: 10.1590/S1677-55382010000500012
ABDOL-MOHAMMAD KAJBAFZADEH, LIDA SHARIFI-RAD, SEYEDSAEID DIANAT
Pediatric Urology Research Center, Department of Pediatric Urology (AMK, SSD) and Department of Physical Therapy (LSR), Children’s Hospital, Tehran University of Medical Sciences, Islamic Republic of Iran
ABSTRACT
Purpose:
To investigate the efficacy of transcutaneous functional electrical stimulation
(FES) on voiding symptoms in children with myelomeningocele (MMC) suffering
from neuropathic urinary incontinence.
Materials and Methods: Six girls and 6 boys
with moderate to severe urinary incontinence secondary to MMC were included.
Median age of children was 5.04 (range: 3-11) years. They underwent a
urodynamic study (UDS) before and 3 months after FES with special attention
to detrusor leak point pressure (DLPP) and maximal bladder capacity (MBC).
Daily incontinence score, frequency of pad changing, and enuresis were
also assessed before and three months after treatment. Fifteen courses
of FES for 15 minutes 3 times per week were performed with low frequency
(40 Hz) electrical current, duration of 250µs, with hold and rest
time of 2 seconds.
Results: Nine children had improvement on
urinary incontinence score, while three children had no improvement. Median
DLPP was significantly increased from 38.5 (range: 12-50) cm H2O to 59.5
(range: 18-83) cm H2O (P = 0.003). MBC was significantly increased from
median value of 155 (range: 60-250) mL to 200 (range: 110-300) mL (P =
0.007).
Conclusions: This is a pilot study showing
that FES therapy might have positive effects on improvement of voiding
symptoms of MMC children with neurogenic urinary incontinence in terms
of daily incontinence score and UDS parameters.
Key
words: myelomeningocele; functional electrical stimulation; urinary
incontinence
Int Braz J Urol. 2010; 36: 614-20
INTRODUCTION
Myelomeningocele
(MMC) is the most common cause of neurogenic bladder in children. Bladder
function in these children is affected by disordered innervation of detrusor
muscle and external urethral sphincter that may lead to hydronephrosis
or reflux and finally renal failure with life-threatening consequences
(1). Treatment of urinary system dysfunction is primarily aimed at preventing
upper urinary tract damage and secondarily at gaining continence and improving
quality of life and social interactions (2). Initial treatment is based
on clean intermittent catheterization (CIC) and anticholinergic medications.
In those who fail to respond to medical treatment, surgical procedures
might be needed (3).
Electrical stimulation has been used for
the treatment of urinary incontinence in adults for several decades and
recently in children (4,5).
Most authors believe that nonimplanted electrical stimulation induces
action potential in the afferent fibers of pudendal nerve leading to efferent
outflow causing contraction of the striated pelvic floor musculature.
In addition, inappropriate detrusor activity might be inhibited by this
modality (6).
Functional electrical stimulation (FES)
is the application of electrical current to the excitable tissue to improve
function that is lost in neurologically impaired individuals. It is a
useful noninvasive therapeutic option that is used as a conservative treatment
with positive results in bladder overactivity (7).
Many patients with urge, stress and mixed
urinary incontinence have been treated with FES using anal or vaginal
electrodes which resulted in inhibitory reflexes against spontaneous detrusor
contraction (8).
To our knowledge, there has been no reported
study evaluating the effects of transcutaneous functional electrical stimulation
on urinary incontinence and urodynamic study (UDS) parameters in MMC children.
This therapeutic option was used in form of a pilot study to improve urinary
incontinence symptoms in MMC patients.
MATERIALS AND METHODS
Between
August 2007 and March 2009, 12 children (6 boys and 6 girls) with neuropathic
urinary incontinence secondary to MMC who were referred to our clinic
at Children’s Hospital Medical Center, Tehran University of Medical
Sciences, were enrolled in the present study. This study was approved
by the local Ethics Committee and written informed consent was obtained
from all children’s caregivers. Inclusion criteria were defined
as children with MMC, aged more than 3 years and moderate to severe urinary
incontinence with unsatisfactory response to conventional treatment (requiring
CIC every 3 to 4 hours and use of pads). Urological evaluation consisted
of renal ultrasonography, urinalysis and UDS. Urodynamic parameters including
mean bladder capacity (MBC) and detrusor leak point pressure (DLPP), were
recorded according to recommendations by International Children’s
Continence Society (ICCS) (9).
Daily incontinence score, the episodes of
nighttime wetting (the number of nights that the child involuntarily micturates
during sleep in a one-week period) and frequency of pad changing (the
number of leakage episodes between two consecutive CICs) were recorded
in a voiding diary by parents. The daily incontinence score was recorded
on a 0-3 scale, as described by Schurch et al. score 0, completely dry;
1, wet once a day, usually at night (mild); 2, wet for < 50% of the
time between CIC (moderate); and 3, wet for > 50% of the time between
CIC (severe) (10). A decrement of 2 or more degrees in the daytime incontinence
score was considered as “improvement”.
UDS (F.M. Wiest Medizintechnik GmbH, Unterhaching,
Germany) was performed according to recommendations by the ICCS in all
patients in a supine position (9). The intravesical and abdominal pressures
were measured simultaneously with a double lumen catheter and with a rectal
balloon catheter. EMG was recorded with superficial electrodes in the
perineal area. Special attention was given to detrusor leak point pressure
(DLPP) and maximal bladder capacity. The same protocol was used for the
UDS performed three months after FES courses. Anticholinergic medications
were discontinued at least seven days prior to both UDS sessions. Subjective
success was assessed by voiding diary and was compared to objective measurements
of UDS.
Following the pretreatment UDS, conventional
treatment (anticholinergic and CIC) was continued and children received
15 courses of transcutaneous FES for 15 minutes in each session, 3 times
per week.
The same electrical stimulation device (model 755X, one-channel NOVIN,
Isfahan, Iran) was used for all the patients. Stimulation was delivered
with an adjustable power setting. Two rectangular self-adhesive (2.5 ×
2.5 cm) electrodes were used. Positive electrode was placed on the skin
above the pubic symphysis, and the negative one was placed on the skin
under urethra.
In all treatment sessions, we used 40 Hz
frequency (to cover both the irritative and obstructive symptoms and stimulate
striated muscle fibers and urethral sphincter in pelvic floor), duration
of 250µs with hold and rest time of 2 seconds. The intensity was
increased until the child experienced a strong but comfortable level of
muscle contractions. Maximum current intensity was below the pain threshold
and well tolerated by the children. In younger children, an intensity
setting of < 30 mA was used. Median current intensity in others was
40 (range: 20-65) mA.
Children were followed for more than 3 months.
All patients underwent UDS three months after the 15 courses of FES.
Statistical analysis was performed by SPSS
16.0 software (SPSS Inc., Chicago, IL). The Wilcoxon-Signed rank test
was executed for non parametric statistical comparisons before and after
treatment. P value of less than 0.05 was considered statistically significant.
RESULTS
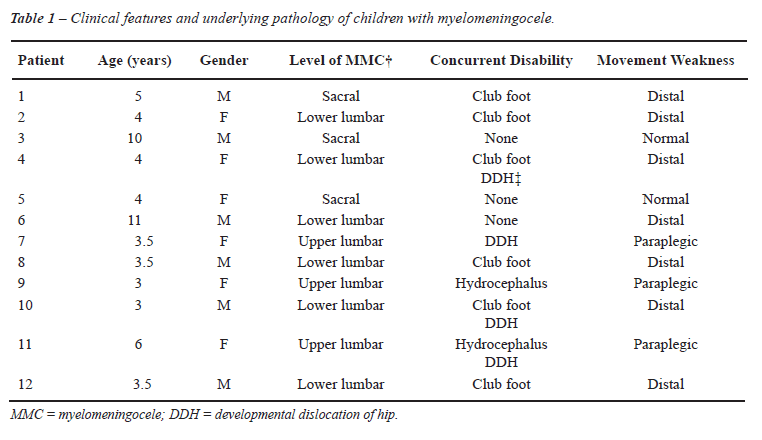
Twelve MMC children including six girls and six boys were enrolled in the present study. Median age of the patients was 5.04 (range: 3-11 years). Demographic data of children are described in Table-1.
Two of twelve patients became completely
dry between two consecutive CICs. Daily incontinence score was improved
from 3 to 1 in five children. Three children remained unchanged for their
daily incontinence score. Of those three children who failed to respond
to electrical stimulation, two were totally incontinent (Daily incontinence
score: 3) initially before the treatment.
Details of voiding characteristics and UDS
parameters before and after FES therapy are summarized in Table-2. Overall,
median daily incontinence score was improved from 3 (range: 2-3) to 1
(range: 0-3) (P = 0.006). Median frequency of pad changing was significantly
decreased from 6 (range: 2-8) to 2 (range: 0-7) times/day (P = 0.004).
Median episodes of night wetting was 3 (range: 2-7) night/week before
the electrical stimulation, which improved to 2 (range: 1-7) night/week
after the treatment (P = 0.06).

Both of two UDS parameters were significantly
improved after treatment. Median DLPP was significantly increased from
38.5 (range: 12-50) cm H2O to 59.5 (range: 18-83)cm H2O (P = 0.003). MBC
was significantly increased from median value of 155 (range: 60-250) mL
to 200 (range: 110-300) mL (P = 0.007). No significant adverse effect
was reported by the children and their parents after treatment.
COMMENTS
Myelomeningocele
repair is still challenging in the literature. Unfavorable effects of
prenatal intervention on postnatal bladder function include poor compliance,
poor detrusor contractility, detrusor-sphincter dyssynergia, hydronephrosis
and vesicoureteral reflux. This may highlight the existence of bladder
developmental defects in these children (11).
In patients with MMC, hyperactivity or inactivity
of either detrusor or external urethral sphincter leads to bladder-sphincter
dysfunction and ultimately urinary incontinence and poor quality of life.
In most of the children, urinary continence can be gained with bladder
emptying by CIC and anticholinergic medication.
Continent catheterizable urinary diversion
is applied in patients who do not respond to anticholinergic medications.
However, it may be complicated by urinary tract infection, distal dehiscence
of conduit, stomal stenosis, and urinary stomal leakage (12).
Mini-invasive collagen sling has also been
used as a safe and easy method with promising immediate results in patients
with neurogenic urinary incontinence. However, a one-year follow-up study
failed to demonstrate beneficial long term outcome (13).
Electrical stimulation, as a clinical non-invasive
treatment option to manage the urinary incontinence symptoms, was first
introduced by Caldwell and colleagues (14).
Numerous electrical stimulation methods
have been reported to be effective for the treatment of lower urinary
tract dysfunction (15).
There are many evidences that electrical
stimulation can lead to activation of detrusor inhibitory reflex as well
as striated urethral muscles contraction (4). This kind of treatment can
cause hypertrophy of muscle fibers, possibly by the recruitment of motor
units with faster conduction and alter the expression of myosin isoforms,
favoring a conversion to type l muscle fibers (4). An effect of FES at
the peripheral level can be modulation of neurotransmitters such as cholinergic
and ß-adrenergic system (16). In a study by Ishigooka et al., reduction
in norepinephrine content of the rabbit urinary bladder by a combination
of yohimbine and electrical stimulation of pelvic floor musculature has
been reported. These authors have suggested the reflexive activation of
hypogastric nerves following pelvic floor stimulation (17). This finding
can describe the effect of FES therapy in patients with bladder overactivity.
Therapeutic effects of FES may be achieved
through normalization and balance between cholinergic and beta-adrenergic
neurotransmitters (8). The prolonged intravaginal FES restores the normal
reflex pattern of detrusor function through reorganization of the neural
system innervating the bladder (18).
Results of our present pilot study revealed
that transcutaneous FES of striated urethral sphincter decreased daily
incontinence score, number of enuresis, and frequency of pad changing
in 75% of MMC patients. In addition, significant improvement obtained
in the UDS parameters (DLPP, MBC) three months after the treatment. We
used this form of stimulation to strengthen the striated urethral muscles
and to normalize voiding pattern with activation of afferent fibers of
pudendal nerve in the perineal region.
We have yielded improvement in urinary symptoms
of children with MMC applying FES in the present study, which was similar
to the results of our previous study in MMC children. In our previous
study, we used interferential electrical current to decrease urinary incontinence
symptoms in MMC children with bladder overactivity: in which 78% of patients
gained continence immediately after treatment and 60% of patients remained
continent for 6 months or more (15).
To our knowledge, there is no report on
the efficacy of FES on urinary symptoms in MMC children. There are several
studies investigating therapeutic effects of FES on urinary incontinence
among adult female subjects.
Primuse and Kramer reported effects of FES
treatment using intravaginal or intra-anal electrodes in 75 patients with
complaints of urgency and/or urge incontinence (30 multiple sclerosis
and 45 idiopathic patients). In these patients, 59% experienced significant
urodynamic and subjective improvement after the treatment and additional
40% of the patients had only subjective improvement of urinary symptoms.
Therapeutic effects of electrical stimulation remained for at least 2
years in 64% of patients with idiopathic urinary incontinence while early
symptom relapse occurred 2 months after the treatment in multiple sclerosis
group (8).
In a study by Hung et al., the effect of
FES-biofeedback and pelvic floor muscle exercise on symptoms of women
with genuine stress urinary incontinence has been investigated. They have
reported that the level of discomfort in daily life, social activity,
physical activity, and personal relations due to urinary symptoms had
significantly improved especially in the FES-biofeedback group (19).
In a study by Kralj et al., effect of FES
on female urinary incontinence has been evaluated. They have reported
50.5% cure, 23.4% improvement of symptoms, and 26.1% treatment failure
three months after the treatment (20).
Eskiyurt et al., have compared the effectiveness
of two therapeutic method including functional magnetic stimulation (FMS)
and functional electrical stimulation (FES) in 22 women with mixed urinary
incontinence. Urinary diaries and micturition frequency was more cured
and improved in those treated by FES than FMS. However, there was no significant
reduction of nocturnal voiding frequency in both groups (21).
There are several limitations in our present
study including small sample size, lack of sham-controlled group, and
short duration of follow-up. It will be necessary to design future studies
to investigate the role of FES therapy in children with myelomeningocele
and compare its effect in a sham-controlled design.
CONCLUSIONS
This type of electrical stimulation is an effective and inexpensive therapeutic method for urinary incontinence in children affected by myelomeningocele with no considerable adverse effects and can be used at home. Applying transcutaneous electrodes makes this type of electrical stimulation a less invasive therapeutic method than anal or vaginal electrode and seems to be better tolerated by children.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Wu HY, Baskin LS, Kogan BA: Neurogenic bladder dysfunction due to myelomeningocele: neonatal versus childhood treatment. J Urol. 1997; 157: 2295-7.
- Nijman RJ: Neurogenic and non-neurogenic bladder dysfunction. Curr Opin Urol. 2001; 11: 577-83.
- Kajbafzadeh AM, Chubak N: Simultaneous Malone antegrade continent enema and Mitrofanoff principle using the divided appendix: report of a new technique for prevention of stoma complications. J Urol. 2001; 165: 2404-9.
- Fehrling M, Fall M, Peeker R: Maximal functional electrical stimulation as a single treatment: is it cost-effective? Scand J Urol Nephrol. 2007; 41: 132-7.
- Malm-Buatsi E, Nepple KG, Boyt MA, Austin JC, Cooper CS: Efficacy of transcutaneous electrical nerve stimulation in children with overactive bladder refractory to pharmacotherapy. Urology. 2007; 70: 980-3.
- Brubaker L: Electrical stimulation in overactive bladder. Urology. 2000; 55(5A Suppl): 17-23; discussion 31-2.
- Bower WF, Yeung CK: A review of non-invasive electro neuromodulation as an intervention for non-neurogenic bladder dysfunction in children. Neurourol Urodyn. 2004; 23: 63-7.
- Primus G, Kramer G: Maximal external electrical stimulation for treatment of neurogenic or non-neurogenic urgency and/or urge incontinence. Neurourol Urodyn. 1996; 15: 187-94.
- Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, et al.: The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006; 176: 314-24.
- Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D: Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000; 164: 692-7.
- Swana HS, Sutherland RS, Baskin L: Prenatal intervention for urinary obstruction and myelomeningocele. Int Braz J Urol. 2004; 30: 40-8.
- Barbosa LL, Liguori R, Ottoni SL, Barroso U Jr, Ortiz V, Macedo A Jr: Is continent urinary diversion feasible in children under five years of age? Int Braz J Urol. 2009; 35: 459-66.
- Taskinen S, Fagerholm R, Rintala R: Mini-invasive collagen sling in the treatment of urinary incontinence due to sphincteric incompetence. Int Braz J Urol. 2007; 33: 395-400; discussion 400-6.
- Caldwell KP: The electrical control of sphincter incompetence. Lancet. 1963; 2: 174-5.
- Kajbafzadeh AM, Sharifi-Rad L, Baradaran N, Nejat F: Effect of pelvic floor interferential electrostimulation on urodynamic parameters and incontinency of children with myelomeningocele and detrusor overactivity. Urology. 2009; 74: 324-9.
- Okada N, Igawa Y, Ogawa A, Nishizawa O: Transcutaneous electrical stimulation of thigh muscles in the treatment of detrusor overactivity. Br J Urol. 1998; 81: 560-4.
- Ishigooka M, Hashimoto T, Sasagawa I, Nakada T: Reduction in norepinephrine content of the rabbit urinary bladder by alpha-2 adrenergic antagonist after electrical pelvic floor stimulation. J Urol. 1994; 151: 774-5.
- Fall M, Lindström S: Electrical stimulation. A physiologic approach to the treatment of urinary incontinence. Urol Clin North Am. 1991; 18: 393-407.
- Sung MS, Hong JY, Choi YH, Baik SH, Yoon H: FES-biofeedback versus intensive pelvic floor muscle exercise for the prevention and treatment of genuine stress incontinence. J Korean Med Sci. 2000; 15: 303-8.
- Kralj B: Conservative treatment of female stress urinary incontinence with functional electrical stimulation. Eur J Obstet Gynecol Reprod Biol. 1999; 85: 53-6.
- Bölükbas
N, Vural M, Karan A, Yalçin O, Eskiyurt N: Effectiveness of functional
magnetic versus electrical stimulation in women with urinary incontinence.
Eura Medicophys. 2005; 41: 297-301.
____________________
Accepted
after revision:
February 27, 2010
_______________________
Correspondence
address:
Dr. Abdol-Mohammad Kajbafzadeh
No. 36, 2nd Floor, 7th Street
Saadat-Abad, Ave.
Tehran, 1998714616, Iran
Fax: + 98 21 2206-9451
E-mail: kajbafzd@sina.tums.ac.ir
EDITORIAL COMMENT
Children
with lower urinary tract congenital anomalies such as bladder exstrophy,
myelomeningocele, or posterior urethral valves, can develop complex clinical
pictures consisting in high-pressure/low flow and hypertonic low compliant
bladders (1). These patients often may need surgical treatment (i.e. cystoplasty)
as they often develop resistance to drug treatment.
In children with myelomeningocele, the main aim of treatment is to improve
the functionality of diseased bladders by decreasing the intravesical
pressures, improving bladder compliance, urinary and fecal continence
and patient’s quality of life. Patients with a poorly compliant
bladder may incur renal damage over time and thus an early effective and
conservative management of bladder dysfunction is welcome. Surgical treatment
may be effective, but side effects are not negligible: bladder augmentation
is usually done with gastrointestinal segments, which can lead to metabolic
abnormalities such as acidosis or alkalosis, depending on the segment
used, an increased rate of calculi formation, increased mucus production,
and enhanced risk of malignant disease (2,3).
Kajbafzadeh and colleagues in this issue of International Brazil Journal
of Urology investigated the efficacy of transcutaneous functional electrical
stimulation (FES) in a small group of children with myelomeningocele and
lower urinary tract dysfunction. Over 12 patients, 9 children reported
an improvement on urinary incontinence score, although three children
had no improvement. It is interesting to note that the detrusor leak point
pressure was significantly improved as well as the maximum bladder capacity.
Authors concluded that FES therapy might have positive effects on improvement
of voiding symptoms of children with neurogenic urinary incontinence in
terms of daily incontinence score and urodynamic parameters.
Every conservative strategy to improve lower urinary dysfunction of children
with neurogenic bladder is welcome, but long-term multiparametric (objective
and subjective) follow-up remains the challenge for next generation of
pediatric and adult urologists.
REFERENCES
- Snodgrass WT, Adams R: Initial urologic management of myelomeningocele. Urol Clin North Am. 2004; 31: 427-34.
- McDougal WS: Metabolic complications of urinary intestinal diversion. J Urol. 1992; 147: 1199-208.
- Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC: Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004; 172: 1649-51; discussion 1651-2.
Dr.
Massimo Lazzeri
Department of Urology
San Raffaele Hospital
Vita-Salute University San Raffaele Turro
Milan, Italy