THE
USE OF IMMUNOHISTOCHEMISTRY FOR DIAGNOSIS OF PROSTATE CANCER
(
Download pdf )
Clinical Urology
doi: 10.1590/S1677-55382010000500008
KATIA R. M. LEITE, MIGUEL SROUGI, ADRIANA SANUDO, MARCOS F. DALL’OGLIO, ADRIANO NESRALLAH, ALBERTO A. ANTUNES, JOSE CURY, LUIZ H. CAMARA-LOPES
Laboratory of Medical Investigation (KRML, MS, AS, MFDO, AN, AAA, JC), Division of Urology, University of Sao Paulo Medical School, and Genoa Biotechnology (KRML, LHCL), Sao Paulo, Brazil
ABSTRACT
Purpose:
Atypical glands (ASAP) are diagnosed in 5.0% of prostate biopsies, and
cancer identification in a rebiopsy is higher than 40.0%. The use of antibodies
to mark basal cells is currently a common practice, in order to avoid
rebiopsies. There has been no reported study that has reviewed characteristics
of radical prostatectomies (RPs) when immunohistochemistry (IHC) was necessary
for definitive diagnosis.
Materials and Methods: Out of 4127 biopsies examined from 2004 to 2008,
144 (3.5%) were diagnosed with ASAP. IHC was performed using antibody
anti-34ßE12 and p63. The results of surgical specimens of 27 patients
treated by RP after the diagnosis of prostate cancer (PC) was made using
IHC (Group 1) were compared with 1040 patients where IHC was not necessary
(Group 2).
Results: IHC helped to diagnose PC in 103 patients (71.5%). Twenty-seven
(26.2%) underwent RP. In Group 1, two (7.4%) adenocarcinomas were insignificant
versus 29 (2.9%) for Group 2. Patients from Group 1 were younger (p =
0.039), had lower Gleason scores (GS) (p < 0.001), lower percentage
of Gleason pattern 4 (p < 0.001), and smaller tumors (p < 0.001).
Conclusion: The use of IHC did not lead to diagnosis of insignificant
tumors as illustrated by absence of differences in pathological stage
or positive surgical margins in men submitted to RP. Therefore, our results
suggest that this modality should be routinely used for a borderline biopsy
and ASAP cases.
Key
words: prostatic neoplasms; diagnosis; biopsy; immunohistochemistry;
atypical small acinar proliferation
Int Braz J Urol. 2010; 36: 583-90
INTRODUCTION
Atypical
glands suspicious for carcinoma, also denominated atypical small acinar
proliferation (ASAP), is not a specific entity but represents a large
group of lesions which includes lesions that mimic cancer and, most importantly,
carcinomas that lack all the cytological and/or architectural characteristics
for the establishment of a definitive diagnosis of cancer. The frequency
of this diagnosis is variable from 0.7 to 23.4%, with a mean of 5.0%,
as reviewed by Epstein and Herawi (1). The possibility of diagnosing cancer
in a subsequent biopsy is high, mean 40.2% (1-3). After radical prostatectomy
(RP), the majority of cases are determined to be low grade and organ-confined
(2,4,5).
In 1984, Gown and Vogel (6) reported the use of a monoclonal antibody
anti-high molecular weight cytokeratin (34ßE12) to mark basal cells
of the prostate that was later demonstrated as a characteristic of benign
glands that retain the basal cell layer (7-9). In a larger series, Wojno
and Epstein (10) used 34ßE12 to diagnose adenocarcinoma in suspicious
glands identified in needle prostate biopsies. Shah et al. (11) later
proposed the combined use of p63, an homolog of the p53 tumor suppressor
protein, as an auxiliary for the determination of cancer since it is also
a protein expressed selectively by the basal cells of epithelial organs,
including the prostate gland (12,13).
Recently, lower levels of prostate specific antigen (PSA) have been used
to indicate the need for a prostate biopsy, and there has been an increasing
number of cores taken in each biopsy session. These new practices have
resulted in the representation of smaller tumors, often more adequately
named ASAP. In addition, pathologists frequently use immunohistochemistry
to enhance their diagnostic capabilities in order to avoid rebiopsies.
There have been reports of false-positives and false-negatives for use
of the combined 34ßE12 and p63 cocktail. To date there has been
no reported study that reviews surgical specimens of tumors from patients
who underwent RP after a diagnosis of carcinoma in which the use of immunohistochemistry
was necessary for the definitive diagnosis.
MATERIALS AND METHODS
From January
1st 2004 to July 31st 2008, 4127 biopsies were examined in our laboratory.
ASAP was the diagnosis made for 144 (3.5%) of the biopsies. The mean age
of the patients was 60.8 years old, median 60 ranging from 40 to 84 years
old. Mean PSA was 7.11 ng/mL, with a median of 5.3 ng/mL, ranging from
1.4 to 43.5 ng/mL. The free to total relation of PSA was mean 15.1%, median
13.0%, ranging from 1 to 30%. The major reason for a biopsy among these
patients was a progressive increase in PSA levels. One patient had a familiar
history, and two had shown abnormalities in transrectal examination. In
31 patients (21.5%), ultrasound examination found abnormalities, hypoecoic
and hypervascular areas. Twenty patients (13.9%) had previously undergone
biopsies. The mean number of cores taken per biopsy session was 15.8,
median 14, ranging from 6 to 40. All the slides were examined by the same
uropathologist.
The immunohistochemistry was performed using a mouse monoclonal antibody
anti-high molecular weight cytokeratin (clone 34ßE12, Dako, Glostrup,
Denmark) at a dilution of 1/100 and p63 (clone 4A4, Dako, Glostrup, Denmark)
at a dilution of 1/100. After paraffin removal and hydration, the slides
were immersed in 10 mM citrate buffer pH 6, for 15 min for antigen retrieval.
The antibodies were incubated overnight at 4°C, and the secondary
biotin-labeled antibody was incubated for 30 min at room temperature.
The streptavidin labeled streptavidin-biotin amplification method (Dako
K0679) was carried out for 30 minutes followed by peroxidase/diaminobenzidine
substrate/chromagen. The slides were counterstained with hematoxylin.
RP was carried out in 27 out of 144 patients. The results were compared
to 1040 patients who underwent RP during the same period and where the
histology was sufficient to define adenocarcinoma. All patients were treated
by the same group of surgeons. The surgical specimens were routinely examined
in toto by the same pathologist. To measure tumor volume we used the grid
method as described by Humphrey and Vollmer (14).
RESULTS
Immunohistochemistry permitted the definitive diagnosis of prostate cancer in 103 patients (71.5%) (Figure-1). The mean age of patients was 61.7 years old, median 61 (range 43 - 84); mean PSA was 7.6 ng/mL, median 5.4 ng/mL (range 1.4 - 44 ng/mL); and PSA free to total ratio was mean 15%, median 13% (range 1 - 29%). The mean Gleason score was 5.9, median 6 (range 4 to 7). The mean and median number of cores positive for tumor was 1.4 and 1 respectively, ranging from 1 to 4. The higher percentage of a single core that made up the tumor was mean 18.3%, median 10% (range 1 to 80%). The mean total percentage of tumor in all cores was 1.6%, median 1% (range 1 to 7%). Perineural invasion was not detected in any cases.

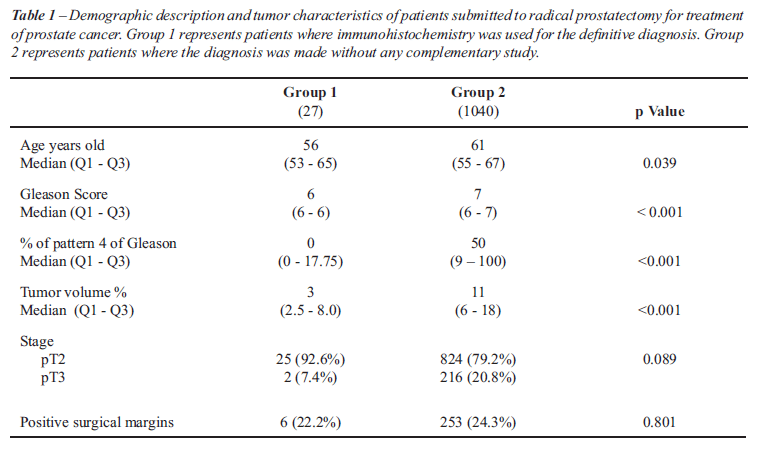
We had access to the surgical specimens
from 27 (26.2%) patients who underwent radical prostatectomy. For the
same period, from January 1 2004 to July 31 2008, 1040 patients also underwent
radical prostatectomy after a diagnosis of adenocarcinoma that did not
necessitate immunohistochemistry for the final diagnosis. The former will
be referred to as Group 1, and the control group as Group 2. The characteristics
of both groups are shown in Table-1. There were no pT0 in any of the two
groups. Within Group 1, two (7.4%) adenocarcinomas could be considered
clinically insignificant, defined as less than 2% of the gland involved,
organ-confined and with no Gleason 4 or 5 pattern present, versus 29 (2.9%)
insignificant cases in Group 2 (15).
The patients in Group 1 were younger, had
lower Gleason scores, a lower percentage of Gleason pattern 4 and smaller
tumors. However, the rate of positive surgical margins was similar and
there were no differences in pathological stage.
COMMENTS
The use
of IHC as an auxiliary in the diagnosis of adenocarcinoma is a common
practice in uropathology, and the use of antibodies against p63 and high
molecular weight cytokeratin has been recommended as adjuncts in confirming
prostatic carcinoma in doubtful cases.
Although basal cell markers, such as 34ßE12 and p63 antibodies are
useful for identifying basal cells, several benign mimickers of PC, such
as atrophy, atypical adenomatous hyperplasia, nephrogenic adenoma, and
mesonephric hyperplasia, can stain negatively with these markers. In addition,
with patients being submitted to prostate biopsy with lower PSA levels
and with larger numbers of cores being taken in each biopsy session, concern
that patients are being overtreated is common.
Our results show that there was no overdiagnosis of PC with any pT0 after
radical prostatectomy, with only 7.4% of cases classified as clinically
insignificant. Tumor stage was similar for both groups, but only 7.4%
of patients from Group 1 had stage pT3 tumors. On the other hand, positivity
of surgical margins, a very important parameter related to the outcome
of patients submitted to radical prostatectomy, mainly in organ-confined
tumors (16,17), was similar for both groups; 22.2% and 24.3% in groups
1 and 2, respectively.
This is the first study to our knowledge to show histological characteristics
of radical prostatectomy specimens in men submitted to surgery to treat
adenocarcinoma where was necessary to use IHC for final diagnosis.
CONCLUSION
Our data show that the usage of IHC did not lead to diagnosis of insignificant tumors, as demonstrated by the study of the radical prostatectomy specimens that had similar pathological stage and positive surgical margins rates. Therefore, our results show that this modality should be routinely used to evaluate a borderline biopsy and ASAP cases.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Epstein JI, Herawi M: Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006; 175: 820-34.
- Leite KR, Mitteldorf CA, Camara-Lopes LH: Repeat prostate biopsies following diagnoses of prostate intraepithelial neoplasia and atypical small gland proliferation. Int Braz J Urol. 2005; 31: 131-6.
- Schlesinger C, Bostwick DG, Iczkowski KA: High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation: predictive value for cancer in current practice. Am J Surg Pathol. 2005; 29: 1201-7. Erratum in: Am J Surg Pathol. 2005; 29: 1548.
- Hoedemaeker RF, Kranse R, Rietbergen JB, Kruger AE, Schröder FH, van der Kwast TH: Evaluation of prostate needle biopsies in a population-based screening study: the impact of borderline lesions. Cancer. 1999; 85: 145-52.
- Iczkowski KA, Bassler TJ, Schwob VS, Bassler IC, Kunnel BS, Orozco RE, et al.: Diagnosis of “suspicious for malignancy” in prostate biopsies: predictive value for cancer. Urology. 1998; 51: 749-57; discussion 757-8.
- Gown AM, Vogel AM: Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984; 114: 309-21.
- Brawer MK, Peehl DM, Stamey TA, Bostwick DG: Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res. 1985; 45: 3663-7.
- Hedrick L, Epstein JI: Use of keratin 903 as an adjunct in the diagnosis of prostate carcinoma. Am J Surg Pathol. 1989; 13: 389-96.
- O’Malley FP, Grignon DJ, Shum DT: Usefulness of immunoperoxidase staining with high-molecular-weight cytokeratin in the differential diagnosis of small-acinar lesions of the prostate gland. Virchows Arch A Pathol Anat Histopathol. 1990; 417: 191-6.
- Wojno KJ, Epstein JI: The utility of basal cell-specific anti-cytokeratin antibody (34 beta E12) in the diagnosis of prostate cancer. A review of 228 cases. Am J Surg Pathol. 1995; 19: 251-60.
- Shah RB, Zhou M, LeBlanc M, Snyder M, Rubin MA: Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am J Surg Pathol. 2002; 26: 1161-8.
- Barbareschi M, Pecciarini L, Cangi MG, Macrì E, Rizzo A, Viale G, et al.: p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001; 25: 1054-60.
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, et al.: p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000; 157: 1769-75.
- Humphrey PA, Vollmer RT: Percentage carcinoma as a measure of prostatic tumor size in radical prostatectomy tissues. Mod Pathol. 1997; 10: 326-33.
- Guzzo TJ, Vira MA, Neway W, Hwang WT, Tomaszewski J, VanArsdalen K, et al.: Minimal tumor volume may provide additional prognostic information in good performance patients after radical prostatectomy. Urology. 2007; 69: 1147-51.
- Psutka SD, Feldman AS, Rodin S, Wu CL, McDougal WS: Positive surgical margins do not affect recurrence in patients with T3a prostate cancer. J Urol. 2009; 181 (4Suppl): 776 #abstract 288.
- Perez D, Shental J, Israel A, Salomon L, Abbou CC: Influence of positive surgical margins on adjuvant treatment after radical prostatectomy. J Urol. 2009; 181 (4 Suppl): 776 #abstract 2138.
____________________
Accepted
after revision:
February 18, 2010
_______________________
Correspondence
address:
Dr. Katia Ramos Moreira Leite
Rua Desembargador Eliseu Guilherme 69
São Paulo, SP, 04004-030, Brazil
Fax: + 55 11 3231-2249
E-mail: katiaramos@uol.com.br
EDITORIAL COMMENT
Immunohistochemistry
(IHC) for 34ßE12 and p63 is at present a diagnostic standard for
determining the presence of prostate cancer. It is also used to discriminate
cancer from mimic cancer, when the definite diagnosis is difficult with
conventional microscopic examinations; however, second biopsy is frequently
recommended in practice. Indeed, 34 - 60% patients showing atypical small
acinar proliferation (ASAP) in the primary biopsies were diagnosed with
prostate cancer in repeat biopsy sessions (1,2).
As the authors stressed in the present paper, few studies have intentionally
examined surgical specimens in patients with prostate cancer, who were
primarily diagnosed with so-called atypical glands in previous biopsy
specimens. Also, recent biopsy protocols such as multi-cores or saturation
method has lead to an increase of ASAP (3). The authors elaborated patients’
demographics as well as radical prostatectomy specimens, of which preoperative
diagnosis in biopsy cores required 34ßE12 and p63 IHC, to underscore
the characteristics and outcome of this type of prostate cancer.
It is of interest that the patients in the IHC-required group were younger,
had lower Gleason score and lower fraction with Gleason pattern 4, and
had smaller tumor foci, compared with those in the IHC-unnecessary group.
These facts may be relevant to lead-time bias in patients examined during
different era with a different screening protocol, or simply based on
earlier disease in younger patients. Although pathological T-stage and
positive surgical margin rates were not statistically different between
the two groups, the difference in patients’ number between them
possibly explains this contradiction. Also, the IHC-required group was
considered to include good-risk cases, while the fraction of patients
diagnosed with insignificant cancer was not large (7.4%). Positive surgical
margin cases were distributed uniformly between the IHC-required and IHC-unnecessary
groups, suggesting that 34ßE12 and p63 IHC was useful as a preoperative
diagnostics even for patients showing equivocal results in biopsy specimens
with routine histology.
REFERENCES
- Schlesinger C, Bostwick DG, Iczkowski KA: High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation: predictive value for cancer in current practice. Am J Surg Pathol. 2005; 29: 1201-7. Erratum in: Am J Surg Pathol. 2005; 29: 1548.
- Epstein JI, Herawi M: Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006; 175: 820-34.
- Ploussard G, Plennevaux G, Allory Y, Salomon L, Azoulay S, Vordos D, et al.: High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation on initial 21-core extended biopsy scheme: incidence and implications for patient care and surveillance. World J Urol. 2009; 27: 587-92.
Dr.
Noboru Hara
Division of Urology
Dep. of Regenerative and Transplant Medicine
Graduate School of Medical and Dental Sciences
Niigata University, Niigata, Japan
E-mail: harasho@med.niigata-u.ac.jp
EDITORIAL COMMENT
This study
was carried out in order to investigate the value of immunohistochemstry
(IHC) in borderline pathological prostate cancer cases. Immunostaining
has gained an important role in the evaluation of borderline biopsy cases,
especially when high-grade prostate intraepithelial neoplasia (HGPIN)
and atypical small acinar proliferation (ASAP) are present. The predictive
values of ASAP and HPGIN for cancer detection on repeat biopsies are 39
and 23%, respectively (1). Usually, the presence of ASAP in the initial
biopsy tissue requires re-biopsy within 6-12 months considering other
clinical features (age, comorbidities etc.). Although this topic has not
been sufficiently discussed in the literature, it may have a significant
impact on patient management. It has been previously recommended that
a second biopsy should include extensive sampling of the initial atypical
site as well as adjacent ipsilateral and contralateral sites with routine
extended schemes. However, extensive transrectal sampling may increase
the risk of infection (2). Herein the authors demonstrate that imunohistochemical
approach, using monoclonal antibody anti-high molecular weight cytokeratin
and p63, may assist in a more accurate diagnosis of ASAP and, therefore,
may obviate the need for re-biopsies with the potential complications.
The main benefit from this study should be related to diagnosis and pretreatment
judgment.
This study shows comparable tumor stage in both study groups (radical
prostatectomy after IHC diagnosis and control) and recommends the inclusion
of IHC in ASAP cases work-up.
This study contributes to our daily clinical practice and may have major
impact on our judgment in borderline prostate biopsy cases. Similar studies
evaluating novel methods to enhance biopsy-based diagnosis accuracy should
be encouraged in view of the intriguing concept of active surveillance.
This study also leads to a yet another question - does active or passive
surveillance miss cases of significant tumors that should be treated more
aggressively? Future studies are warranted to answer this question.
REFERENCES
- Iczkowski KA: Current prostate biopsy interpretation: criteria for cancer, atypical small acinar proliferation, high-grade prostatic intraepithelial neoplasia, and use of immunostains. Arch Pathol Lab Med. 2006; 130: 835-43.
- Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006; 175: 820-34.
Dr.
Avraham Shtricker
The Sackler School of Medicine
Tel Aviv University
Tel Aviv, Israel
E-mail: shtrickera@hotmail.com
EDITORIAL COMMENT
Authors
compared the pathologic stage between radical prostatectomy cases performed
in patients for which cancer was diagnosed with the help of combined 34ßE12
and p63 cocktail IHC (n = 27) or not (n = 1040). Their results showed
that there was no overdiagnosis of prostate cancer with any stage pT0
after radical prostatectomy, with only 7.4% of cases classified as clinically
insignificant. This shows the interest of IHC staining and the quality
of the pathologist reading.
Pathologists frequently use immunohistochemistry to enhance their diagnostic
capabilities in order to avoid rebiopsies in cases of diagnosis difficulty,
which is a frequent situation in biopsy reading. I would make 2 comments:
1) Recent studies recommend not to use the ASAP definition but atypical
foci
2) It was also demonstrated that a different IHC of a p63/alpha-methyl-CoA-racemase
(p504s) cocktail in case of atypical foci in the prostate has a diagnostic
utility (1-3).
Authors did not use this p504s in their IHC study. It may be discussed
whether the addition of the p504s is superior or not to the 34ßE12
and p63 cocktail authors used.
REFERENCES
- Molinié V, Fromont G, Sibony M, Vieillefond A, Vassiliu V, Cochand-Priollet B, et al.: Diagnostic utility of a p63/alpha-methyl-CoA-racemase (p504s) cocktail in atypical foci in the prostate. Mod Pathol. 2004; 17: 1180-90.
- Iczkowski KA, MacLennan GT, Bostwick DG: Atypical small acinar proliferation suspicious for malignancy in prostate needle biopsies: clinical significance in 33 cases. Am J Surg Pathol. 1997; 21: 1489-95.
- Iczkowski KA: Current prostate biopsy interpretation: criteria for cancer, atypical small acinar proliferation, high-grade prostatic intraepithelial neoplasia, and use of immunostains. Arch Pathol Lab Med. 2006; 130: 835-43.
Dr.
Arnauld Villers
Service d’Urologie
Hôpital HURIEZ, CHRU
Lille, France
E-mail: arnauld.villers@wanadoo.fr
REPLY BY THE AUTHORS
To Dr. Arnauld Villers Comment
ASAP is a term that has been discussed in uropathology meetings, and some pathologists argument that the suspicious gland is not always small, so it should be called atypical focus. ASAP was a very good term coined to describe doubtful lesions, and has been used for a long time. It means for us, pathologists, the presence of a small focus of atypical glands, not necessarily small, highly suspicious for cancer and help pathologists and urologists to communicate. Also, used as a keyword facilitates the search in the literature, whereas atypical has a profuse meaning. Concerning the use of alpha-methyl-CoA-racemase (p504s), it has been shown that it stains number of benign prostate glands, periurethral glands and mimics of prostate cancer (1), increases the cost and help the final decision in only 50% of cases (2).
REFERENCES
- Gologan A, Bastacky S, McHale T, Yu J, Cai C, Monzon-Bordonaba F, Dhir R: Age-associated changes in alpha-methyl CoA racemase (AMACR) expression in nonneoplastic prostatic tissues. Am J Surg Pathol. 2005; 29: 1435-41.
- Zhou M, Aydin H, Kanane H, Epstein JI: How often does alpha-methylacyl-CoA-racemase contribute to resolving an atypical diagnosis on prostate needle biopsy beyond that provided by basal cell markers? Am J Surg Pathol. 2004; 28: 239-43.
The Authors