HAND-ASSISTED
LAPAROSCOPIC NEPHRECTOMY AS A MINIMALLY INVASIVE OPTION IN THE TREATMENT
OF LARGE RENAL SPECIMENS
(
Download pdf )
M. TOBIAS-MACHADO, ALESSANDRO TAVARES, PEDRO H. FORSETO JR, JOAO P. ZAMBON, ROBERTO V. JULIANO, ERIC R. WROCLAWSKI
Section of Urology, ABC School of Medicine, Santo Andre, Sao Paulo, Brazil
ABSTRACT
Introduction:
We describe our experience with hand-assisted laparoscopy (HAL) as an
option for the treatment of large renal specimens.
Materials and Methods: Between March 2000
and August 2004, 13 patients candidate to nephrectomies due to benign
renal conditions with kidneys larger than 20 cm were included in a prospective
protocol. Unilateral nephrectomy was performed in cases of hydronephrosis
(6 patients) or giant pyonephrosis (4 patients). Bilateral nephrectomy
was performed in 3 patients with adult polycystic kidney disease (APKD)
with low back pain refractory to clinical treatment previous to kidney
transplant. The technique included the introduction of 2 to 3 10 mm ports,
manual incision to allow enough space for the surgeon’s wrist without
a commercial device to keep the pneumoperitoneum. The kidney was empty,
preferably extracorporeally, enough to be removed through manual incision.
We have assessed operative times, transfusions, complications, conversions,
hospital stay and convalescence.
Results: The patients mean age (9 women
and 4 men) was 58 years. Mean operating time was 120 ± 10 min (hydronephrosis),
160 ± 28 min (pyonephrosis) and 190 ± 13 min (bilateral
surgery for APKD). There was a need for a conversion in 1 case and another
patient needed a transfusion due to a lesion in the renal vein; 2 patients
had minor complications.
Conclusions: HAL surgery is a minimally
invasive alternative in the treatment of large renal specimens, with or
without significant inflammation.
Key
words: nephrectomy; laparoscopy; pyelonephritis; hydronephrosis;
polycystic kidney, autosomal dominant
Int Braz J Urol. 2005; 31: 526-33
INTRODUCTION
Some
authors have considered the presence of large renal specimens or inflammatory
kidney disease as contraindications regarding the use of laparoscopy.
The difficult access to the kidney limits, the contiguous fibrosis and
the difficult in identifying the renal vessels lead to longer operating
times and higher complication and conversion rates when surgery is performed
through pure laparoscopic technique (1,2).
In 1997, Nakada et al. performed the first
hand-assisted laparoscopic nephrectomy (HAL) in humans (3). Since then,
even if not in a consensual manner, this access has become an alternative
to more difficult nephrectomies.
Some studies in nephrectomy for kidney donation
have demonstrated that HAL can be superior to the open technique and similar
to the exclusive laparoscopic one when we take into consideration postoperative
recovery (4,5). In the case of kidneys with severe inflammation, there
must be a significant reduction in operating times, with minimum differences
in terms of morbidity when we compare HAL with the exclusive laparoscopic
technique (6).
The aim of the present study was to report
the experience in the treatment of 16 giant renal specimens utilizing
HAL, discussing the advantages of this procedure and comparing the results
with literature results.
MATERIAL AND METHODS
Selection
Criteria and Sample Descriptions
In the period from March 2000 to August
2004, 13 patients with giant kidneys (more than 20 cm in the largest diameter
or crossing the midline) candidates of nephrectomy due to benign renal
conditions were submitted to HAL nephrectomy and followed through a prospective
protocol. A computerized tomography of the abdomen was systematically
performed, assessing kidney size, hilum position (frequently altered due
to the distortion because of the exaggerated volume of these kidneys),
and the degree of inflammation, through the perirenal fat smear.
Indications for unilateral nephrectomy were
pyonephrosis in 6 patients (one of them with adult polycystic kidney disease
- APKD) and giant hydronephrosis without a significant inflammatory component
(nonfunctioning kidneys with stenosis of the ureteropelvic junction) in
4 patients. Bilateral nephrectomy was performed in 3 patients bearers
of terminal renal insufficiency due to APKD previously to the kidney transplant,
with low back pain refractory to clinical treatment. All patients with
renal insufficiency were submitted to dialysis the day before the operation.
No colon preparation was utilized, being
employed 1 g of cephalotine as an antibiotics prophylaxis in the anesthetic
induction, repeated every 6 hours in the first 48 h.
Operative
Technique
Two surgeons assisted by resident physicians
in training operated all patients included. For the unilateral nephrectomy,
the patient was placed in a lateral decubitus position with the kidney
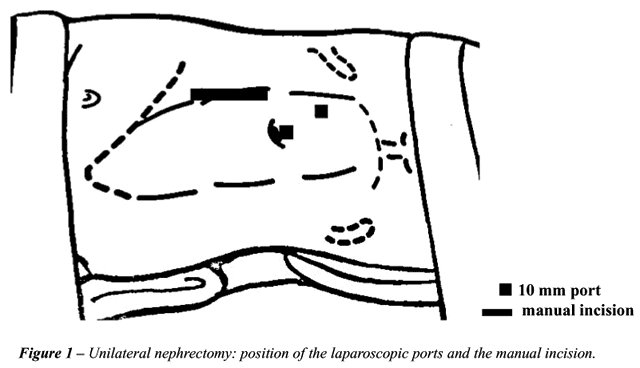
to be operated facing upwards. An internal pararectal incision of approximately
6 cm exact for the surgeon’s wrist and 2 laparoscopic ports were
used (Figure-1). We have planned the incision over the projection of the
renal vessels with the help of a preoperative tomography.
In the cases of bilateral nephrectomy, the
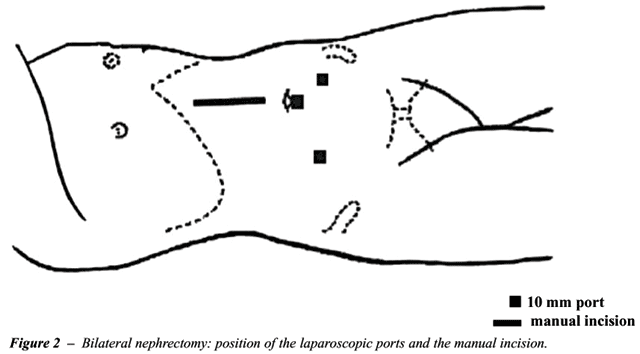
patient was placed in a horizontal dorsal decubitus position, and he/she
was fixed to the table in order to enable its lateral mobilization according
to the side to be approached. One sole incision in the midline was utilized
to both side nephrectomies. Three 10 mm ports were utilized (Figure-2).
To reduce the costs, permanent laparoscopic
material was employed and no commercial device to keep the pneumoperitoneum
was used. The renal pedicle was controlled by a Hem-o-lok® polymer
clip (Weck Closure Systems, Research Triangle Park, NC, USA) proximal
and distal both for the artery and for the vein. In the impossibility
of laparoscopic control of the pedicle, we have placed long Doyen valves
through the manual incision and have performed the individualized extracorporeal
ligature of the vessels. When the presence of inflammation hindered the
individualization of the vessels, we have completely mobilized the kidney
through a manual dissection for a further application of Satinsky clamp,
pedicle section and suture with polypropylene through the previously planned
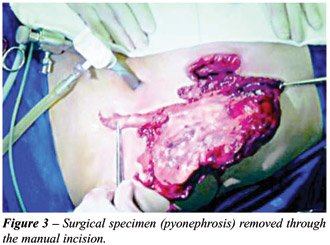
incision. The removal of the specimen was done through the hand incision
(Figure-3), without previously placing it in a sac, after an extracorporeal
drainage (hydronephrosis without infected content) or multiple extracorporeal
punches (pyonephrosis or APKD). The manual incision and the ports aponeurosis
were either closed with polypropylene 0 and the skin with nylon 4-0 (when
the possibility of infection was considered) or with intradermal suture
with monocryl 5-0. In the presence of pyonephrosis, the manual incision
was systematically washed with saline and a Penrose drain was placed through
a 10 mm port.
Patients were followed-up at an outpatient
clinic by the surgeon on days 7, 30, 60 and 90 postoperatively.
RESULTS
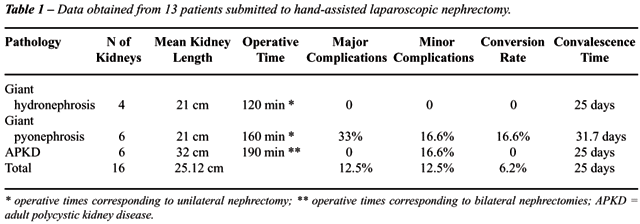
The
main results obtained in this group of patients can be verified in Table-1.
Due to the reduction of working space, there
is a constant need for the surgeon to orient the assistant for a correct
optical direction to the field that we intend to approach.
We have observed that the pneumoperitoneum
can be maintained in all cases. The mean number of times that the hand
had to be taken out the cavity was 2 (1 to 4) times per kidney, mainly
for the exchange of compresses or for the performance of external maneuvers.
Since there was no mechanism to contain the gas, there was a need to wait
until insufflation was renewed to restart surgery. This happened without
any harm to the procedure. The compression of the surgeon’s wrist
through the aponeurosis was not troublesome when the surgery for the kidney
occurred in up to 2 hours. The control of the renal pedicle was obtained
through exclusive laparoscopic maneuvers in 11 kidneys. In 3 kidneys (pyonephrosis),
the application of a Satinsky clamp was needed after the whole kidney
was mobilized. In a patient with APKD, we have chosen the ligature and
section of the vessels of the 2 kidneys after external and bilateral individualization
through the manual incision. In these cases, an additional enlargement
of the incision was needed for the control of the renal pedicle.
Operative times were 120 ± 10 min
(hydronephrosis), 160 ± 28 min (pyonephrosis) and 190 ±
13 min (APKD).
No case of simple hydronephrosis presented
complications. Two cases presented major complications (vascular lesion,
one with transfusion and another followed by conversion) and 2 cases minor
complications (asymptomatic pneumothorax and wound infection, followed
by hernia).
A patient with pyonephrosis presented a
lesion in the right renal vein during the manual displacement of the kidney.
The lesion was tamponade with the help of a hand and controlled with a
polymer clip. Patient received intraoperatively 2 red blood cell concentrates
without the need for conversion, being discharged from the hospital in
the third day postoperatively.
One of the APKD patients developed a right
pneumothorax, drained in the immediate postoperative. The drain was taken
out after 48 hours and the patient was dismissed in the 4th postoperative
day without any complication.
One of the patients with morbid obesity
and pyonephrosis developed a surgical wound infection, satisfactorily
treated with local care. In the late follow-up, he presented an incisional
hernia surgically corrected with a Marlex mesh.
In one of the cases, a conversion to open
surgery was necessary. This 72 year-old patient presented APKD and a clinical
picture of a recurrent right pyelonephritis. During the parietocolic gutter
displacement, we have observed an intense adhesion to the right colon,
impeding access to the renal pedicle in the initial operative time. The
dimension of this kidney that overcame the midline, promoted an additional
difficulty to the endoscopic procedure, ending up in a tactical conversion
through an enlargement of the manual incision, from the costal edge to
the right iliac fossa. Total operative time was 192 min, being discharged
in the 7th postoperative day. The return to normal activities occurred
65 days after surgery.
The other patients were discharged between
the 1st and the 5th postoperative day (mean 3.4 days). Thirty days after
the surgery (mean 25 days), all patients, exception made to the case that
needed conversion, were totally recovered to normal activities.
COMMENTS
Laparoscopic
renal surgery is the gold standard treatment for renal ablation of benign
non-inflammatory pathology. Even though, in some cases of large renal
specimens or associated to intense inflammatory process, renal laparoscopic
surgery still presents some restrictions. Although this is a subject of
great controversy in literature, some authors report that in these situations,
pure laparoscopic surgery presents operative time, complication and conversion
rates superior to the habitual ones, being the benefits in these cases
not as notorious. On the other hand, the conventional technique in these
conditions frequently requires either a lumbar or a median incision of
large dimensions.
HAL surgery appears as an attractive alternative
in these cases, adding up the advantages of a minimally invasive treatment
to the possibility of a faster and safer surgery. It allows the introduction
of the surgeon’s hand in the operative field, making it easier maneuvers
of dissection, retraction and hemostasia while keeping both tactile and
spatial sensations. In case of a vascular accident, the control can be
easily obtained through a digital compression of the vessel, allowing,
if necessary, a conversion in non-emergency conditions. Besides, one or
more compresses can be introduced in the cavity to tamponade a certain
region while dissection continues (7,8).
We believe that hand-assisted technique
has a precise indication in cases where technical difficulty is previewed.
In kidneys with an accentuated inflammation, Wolf et al. have demonstrated
a significant reduction in operative time comparing HAL with conventional
laparoscopy, with minimum differences in terms of morbidity (6).
Although there are evident advantages as
to the comfort during the procedure, the cost of devices to keep the pneumoperitoneum
during HAL as the Hand Port System® (Smith and Nephew, Inc., Andover,
MA, USA), the Intromit hand assistance device® (Applied Medical, Santa
Margarita, CA, USA), the Lapdisc® (Ethicon Inc., Cincinnati, OH, USA)
or the Pneumo Sleeve® (Dexterity, Roswell, GA, USA), it can be a limiting
factor in most of public services in developing countries. Due to institutional
questions, we utilized HAL without the assistance of special devices.
We could notice that this procedure is feasible though an incision large
enough to fit the surgeon’s wrist, with the maintenance of the pneumoperitoneum,
which is lost only momentarily when there is a need for a large mobilization,
the retrieval or exchange of the surgeon’s hand.
Regarding the surgical indication, it was
demonstrated in this series that the size of the kidney in the absence
of inflammation is not a factor of contraindication to the laparoscopic
procedure. Giant hydronephrosis has been defined as a kidney that has
more than 1000 mL of fluid in its collecting system (9). This group of
patients was submitted to the procedure with a very satisfactory operative
time. In this specific indication, pure laparoscopy can also be performed
safely, however with a longer operative time. Morbidly obese, where pure
laparoscopic surgery can present higher technical difficulty, and patients
with precarious clinical conditions, where a short operative time added
to a minimally invasive surgery is essential, represent 2 sub-groups where
access to HAL can have an additional advantage over the exclusive laparoscopic
access.
Laparoscopic nephrectomy in cases of pyonephrosis
is a highly complex surgery. The difficult visualization of the dissection
plans, the inflammation of the tissue, the adherence of vital structures
(colon, duodenum, liver, gallbladder and great vessels) and the obliteration
of the renal hilum are factors that make this procedure challenging. In
all pyonephrosis cases in this study, kidneys presented more than 20 cm
in the biggest axis and signs of perirenal fat infiltration in the preoperative
computerized tomography. For all these aggravating factors most part of
the procedures can be performed without the need for conversion to the
open procedure. Digital renal dissection in the subcapsular plane and,
eventually, the extracorporeal ligature of the pedicle with a Satinsky
clamp, in face of great difficulties, are alternatives that can avoid
the conversion to the conventional technique.
APKD is a common genetic disorder that is
inherited as an autosomal dominant disease, characterized by multiple
bilateral renal cysts, nonfunctioning and noncommunicating. Nephrectomy
in APKD can be necessary when the native kidney occupies all the iliac
fossa where the kidney will be transplanted, or due to hypertension or
refractory pain, hematuria requiring transfusion and recurrent infections,
symptoms that appear between the third and fourth decades of life. Around
50% of the patients develop terminal renal insufficiency up to 60 years
old, needing dialysis or renal transplant procedures (10). The rate of
complications associated to conventional open procedure is significant,
with 12 % morbidity and 5 % mortality rate (11). These numbers were responsible
for a decrease in nephrectomy for APKD patients between 1970 and 1980
(12,13).
Some authors propose the performance of
nephrectomy in patients with APKD with pure transperitoneal (TP) or retroperitoneal
(RP) laparoscopy. The series of nephrectomies through the TP approach
show that, even through they are feasible technically, operative time
is very long (more than 4 hours for unilateral nephrectomies) (14-16).
As to the RP approach, the need for an extended previous experience in
retroperitonoscopy is described for surgical success. A special disadvantage
of this approach is the need to reposition the patient to access the kidney
in the opposite side, a procedure that requires around 45 min. and that
is not necessary in the cases of TP or HAL approach (17). Recent series
using the HAL technique, reporting a shorter operative time, with less
morbidity and mortality, can rekindle interest for a more precautious
performance of nephrectomies in patients with APKD (7,18,19).
The performance of a bilateral nephrectomy
at once offers some advantages over the two-step procedure, including
the need of only one anesthetic and hospital stay, lesser risk of incisional
complications and manipulation of previous surgery adherences. It is frequently
difficult to define with certainty the side responsible for the origin
of the painful symptoms and in this case it is prudent the simultaneous
removal of both kidneys for a maximum relief of symptoms (17). In some
cases, the performance of a nephrectomy is essential as preparation for
a transplant (20). In our 3 patients with APKD, there was a low back pain
control without the need of pain medicines beyond the 5th postoperative
day. The complications rate of this series of special patients was not
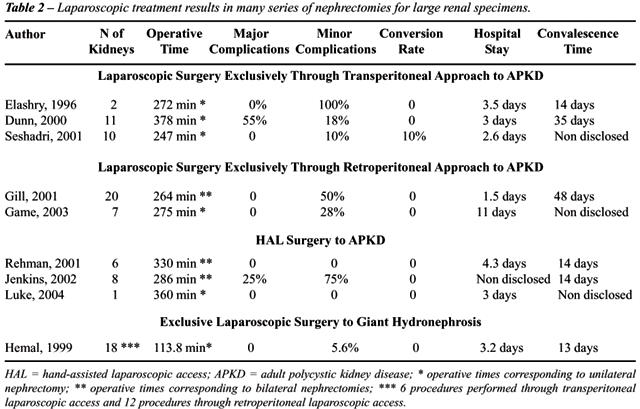
significantly higher than other series of laparoscopic nephrectomy (Table-2)
and, especially, operative time for nephrectomy in APKD patients was shorter
than the one found in literature. Our case of conversion occurred in a
patient with APKD and infected cysts.
CONCLUSIONS
HAL nephrectomy is an attractive alternative to the treatment of large renal specimens in the presence or not of significant inflammation. In cases that would be difficult to perform exclusively through laparoscopy, HAL allows the dissection to be done in a more agile and safer way, keeping the advantages of a minimally invasive treatment.
CONFLICT OF INTEREST
None declared.
REFERENCES
- Tan YH, Siddiqui K, Preminger GM, Albala DM: Hand-assisted laparoscopic nephrectomy for inflammatory renal conditions. J Endourol. 2004; 18: 770-4.
- Tobias-Machado M, Lasmar MT, Batista LT, Forseto Jr PH, Juliano RV, Wroclawski ER: Laparoscopic nephrectomy in inflammatory renal disease: proposal for a staged approach. Int Braz J Urol. 2005; 31: 22-8.
- Nakada SY, Moon TD, Gist M, Mahvi D: Use of the PneumoSleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997; 49: 612-3.
- Wolf JS Jr, Merion RM, Leichtman AB, Campbell DA Jr, Magee JC, Ounch JD, et al.: Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation. 2001; 72: 284-90.
- Stifelman MD, HulL D, Sosa RE, Su LM, Hyman M, Stubenbord W, et al.: Hand assisted laparoscopic donor nephrectomy: a comparison with the open approach. J Urol. 2001; 166: 444-8.
- Wolf JS Jr, Moon TD, Nakada SY: Hand assisted laparoscopic nephrectomy: comparison to standard laparoscopic nephrectomy. J Urol. 1998; 160: 22-7.
- Rehman J, Landman J, Andreoni C, McDougall EM, Clayman RV: Laparoscopic bilateral hand assisted nephrectomy for autosomal dominant polycystic kidney disease: initial experience. J Urol. 2001; 166: 42-7.
- Stifelman MD, Handler T, Nieder AM, Pizzo JD, Taneja S, Sosa RE, et al.: Hand-assisted laparoscopy for large renal specimens: a multi-institutional study. Urology. 2003; 61: 78-82.
- Hemal AK, Wadhwa SN, Kumar M, Gupta NP: Transperitoneal and retroperitoneal laparoscopic nephrectomy for giant hydronephrosis. J Urol.1999; 162: 35-9.
- Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med. 1993; 323: 332-42.
- Brazda E, Ofner D, Riedman B, Spechtenhauser B, Margreiter R: The efect of nephrectomy on the outcome of renal transplantation in patients with polycystic kidney disease. Ann Transplant. 1996; 1: 15-17.
- Lazarus JM, Bailey GL, Hampers CL, Merrill JP: Hemodialisys and transplantation in adults with polycystic kidney disease. JAMA. 1971; 217: 1821-4.
- Ho-Hsieh H, Novick AC, Steinmuller D, Streem SB, Buszta C, Goormastic M: Renal transplantation for end-stage polycystic renal disease. Urology. 1987; 30: 322-6.
- Seshadri PA, Poulin EC, Pace D, Schlachta CM, Cadeddu MO, Mamazza J: Transperitonial laparoscopic nephrectomy for giant polycystic kidneys: a case control-study. Urology. 2001; 58: 23-7.
- Elashry OM, Nakada SY, Wolf JS Jr, McDougall EM, Clayman RV: Laparoscopy for adult polycystic kidney disease: a promising alternative. Am J Kidney Dis. 1996; 27: 224-33.
- Dunn MD, Portis AJ, Elbahnasy AM, Shalhav AL, Rothstein M, McDougall EM, et al.: Laparoscopic nephrectomy in patients with end stage renal disease and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000; 35: 770-2.
- Gill IS, Kaouk JH, Hobart MG, Sung GT, Schweizer DK, Braun WE: Laparoscopic bilateral syncronous nephrectomy for autosomal dominant polycystic kidney disease: the initial experience. J Urol. 2001; 165: 1093-8.
- Luke PP, Spodek J: Hand-assisted laparoscopic resection of the massive autosomal dominant polycystic kidney. Urology. 2004; 63: 369-72.
- Jenkins MA, Crane JJ, Munch LC: Bilateral hand-assisted laparoscopic nephrectomy for autosomal dominant polycystic kidney disease using a single midline handport incision. Urology. 2002; 59: 32-6.
- Game X, Vaessen C, Mouzin M, Mallet R, Malavaud B, Sarramon JP, et al.: Retroperitonial laparoscopic nephrectomy for polycystic kidney: preliminary results. Prog Urol. 2003; 13: 215-21.
___________________
Received: April 6, 2005
Accepted after revision: August 30, 2005
_______________________
Correspondence address:
Dr. Marcos Tobias-Machado
Rua Graúna, 104/131
São Paulo, SP, 04514-000, Brazil
Fax: +55 11 288-1003
E-mail: tobias-machado@uol.com.br