EVALUATING
THE EFFICIENCY OF A COMBINATION OF PYGEUM AFRICANUM AND STINGING NETTLE
(URTICA DIOICA) EXTRACTS IN TREATING BENIGN PROSTATIC HYPERPLASIA (BPH):
DOUBLE-BLIND, RANDOMIZED, PLACEBO CONTROLLED TRIAL
(
Download pdf )
ÉZER A. MELO, EDUARDO B. BERTERO, LUIZ A. S. RIOS, DEMERVAL MATTOS JR.
Division of Urology, Hospital do Servidor Público Estadual de São Paulo, SP, Brazil
ABSTRACT
Objectives:
In spite of its historical use, published data about phytotherapic products
is characterized by the absence of well conducted studies, leading to
conflictive and indefinite results about efficiency and safety of theses
drugs. In that sense, we have analyzed the results of a combination of
Pygeum africanum and stinging nettle (Urtica dioica) extracts in patients
with benign prostatic hyperplasia (BPH), based in a double-blind, randomized,
placebo-controlled protocol.
Materials and Methods: We have selected,
according to inclusion and exclusion criteria, only patients with ³50
years, presenting urinary symptoms assessed by the International Prostatic
Symptoms Score (IPSS), with minimum score of 12, and Quality of Life (QoL)
index of at least 3 points, rectal examination consistent with BPH, and
maximum urinary flow rate (Qmax) between 5 and 15 mL/s. Phytotherapic
and placebo groups were formed by 27 and 22 patients, respectively. The
major variables analyzed during the study were IPSS variation, Qmax, and
side effects. Reduction of ³30% and ³50% in IPSS were the parameters
used to define a clinically significant response (CSR). We have also analyzed
³30% and ³50% Qmax increases.
Results: After six months of treatment we
did not observe significant differences in clinical improvement potential
between the phytotherapic combination and placebo groups. Percent IPSS
drop of 21.6% in the phytotherapic group was similar to 19.7% obtained
in the placebo group (p=0.928). Neither we observed any difference (p=0.530)
for QoL improvement between phytotherapic (9.26%) and placebo (5.98%)
groups. The alterations of Qmax followed the trend line observed in clinical
data, with no significant difference (p=0.463) in Qmax increasing percent
between phytotherapic (17.2%) and placebo (13.3%) groups. The CSR evaluation
of clinical and urodynamic data was also similar between the groups.
Conclusion: The combination of 25mg Pygeum
africanum and 300mg stinging nettle extracts produced clinical and urodynamic
effects similar to placebo in a group of HBP patients.
Key words:
prostate; prostatic hyperplasia; phytotherapy; Pygeum africanum
Int Braz J Urol. 2002; 28: 418-25
INTRODUCTION
The
use of phytotherapic drugs for the treatment of benign prostatic hyperplasia
(BPH) patients has a long history, especially in European countries. Nevertheless,
there is still a considerable degree of skepticism from the urologic community
about the efficiency and safety of these products. This is mainly due
to the absence of an established mechanism of action for phytotherapics.
Phytotherapics used in this clinical trial
consisted in a combination of plant extracts with 25mg Pygeum africanum
and 300mg stinging nettle. Stinging nettle presents a complex mixture
of water and alcohol soluble compounds in its composition (1). Its presumed
mechanism of action, tested in experimental animals, is related to the
inhibition of growing factors, suppression of metabolism and growing of
prostatic cell, and modulation of globulin binding to sexual hormone receptor
in cell membrane (2-4).
Pygeum africanum extract is taken
from the bark of the african plum tree. In vitro studies indicate that
the effect of Pygeum africanum would be exerted by the inhibition
of growing factors, anti-inflammatory and anti-estrogenic action (5).
Recently, Levin et al., in experimental studies, suggested that P.
africanum extract could revert or protect bladder dysfunctions secondary
to prostatic obstructive process (6). However, to obtain these functional
effects, we used up to 100mg/kg doses (7). Recommended dose for clinical
use is 100 mg/day.
The objective of this study is to assess
efficiency and safety of 25mg Pygeum africanum and 300mg stinging
nettle extracts in the treatment of BPH patients. To our knowledge, this
is the first clinical trial with this combination of plant extracts where
evaluation criteria recommended by international consensus on BPH according
to MEDLINE and LILACS were used.
MATERIALS AND METHODS
Inclusion
and exclusion criteria used in patients’ selection were already published
previously (8). Were included only the patients ³50 years, with urinary
symptoms assessed by IPSS with minimal score=12, Quality of Life (QoL)
index of at least 3 points, rectal examination consistent with BPH, and
maximum urinary flow rate (Qmax) between 5 and 15 mL/s. The study protocol
was approved by the Committee of Ethics and Research of our hospital,
and each patient signed an informed consent.
The study protocol had 6 months. After initial
visits for selection, each patient was oriented to return in 6 periods
of 4 weeks, totaling 6 return visits, to answer IPSS and assess side effects.
Qmax was measured in the initial visit and after 6 months of treatment.
Prostatic volume and urinary residual post-voiding were determined only
during the initial visit for selection.
Patients
were divided in 2 groups according to a prospective, randomized, and double-blind
protocol: phytotherapy group (PhyG) with 27 patients, and placebo group
(PlaG) with 22 patients. The randomization process was done by the laboratory,
where pills bottles were identified by numbers. At the end of the study
the keys indicating which patients received phytotherapics or placebo
were opened. Each group received 1 PO bid Pygeum africanum 25mg
+ stinging nettle 300 mg or placebo pill during 6 months.
Major
variables analyzed were IPSS observed variation, Qmax, and side effects
during the study. We have defined 2 levels of clinically significative
response (CSR): ³30% and ³50% IPSS drop. We have also analyzed
³30% and ³50% Qmax increases.
STATISTICS
Presence of association among qualitative variables was evaluated through Chi-square (c²) test or Fisher’s exact test, and comparison between both groups regarding quantitative variables was made by Student’s t-Test and Mann-Whitney non-parametric test (U-test). Comparisons between initial and after 6 months of treatment measures, within each group, were made by Wilcoxon’s paired signed rank test (z).
RESULTS
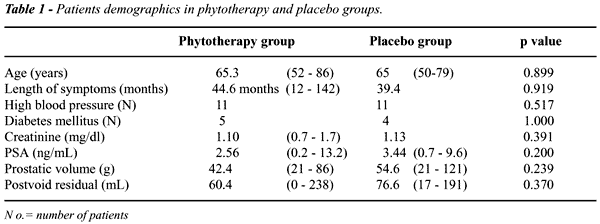
Table-1
show major demographics, clinical, and laboratorial characteristics of
patients selected in each group. Patients mean age was 65 years in both
groups (p=0.899). Length of urinary symptoms was also similar between
the groups (p=0.919).
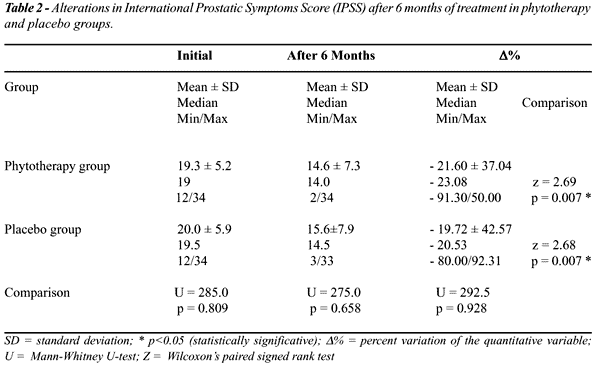
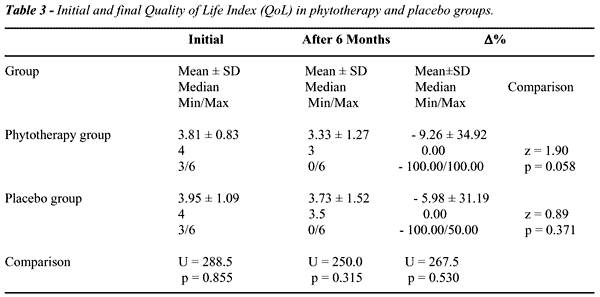
After
6 months of treatment, we did not observe significant differences between
patients receiving Pygeum africanum + stinging nettle combination
and placebo, regarding clinical improvement (Tables-2 and 3). Although
there was a significative drop in IPSS between the groups, we did not
observe differences in the percent variation between the groups at the
end of the treatment (p=0.928). The reduction percent in QoL in PhyG (mean
9.26%) and in PlaG (mean 5.98%) was not significative either after 6 months
of treatment (p=0.530).
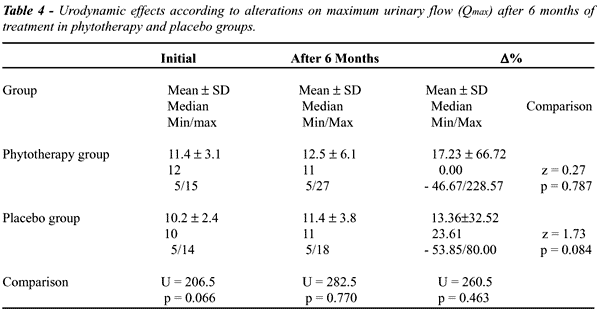
In
Qmax a difference marginally significative was observed between the groups
at the initial evaluation (p=0.066), i.e., there was a trend towards PhyG
patients presenting Qmax greater than PlaG patients (Table-4). However,
even though this trend was verified in PhyG, we did not observe differences
in percent increase between the groups (p=0.463).
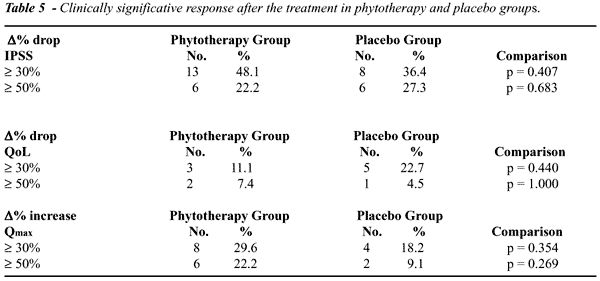
Evaluating
IPSS and QoL percent variation among patients studied, we did not find
statistically significant differences between the 2 groups regarding =30%
(p=0.407 and p=0.440) and =50% (p=0.683 and p=1.000) drop after completion
of the clinical trial. Regarding Qmax, no significant differences between
the groups for =30% (p=0.354) e =50% (p=0.269) increases at treatment
completion were observed either (Table-5).
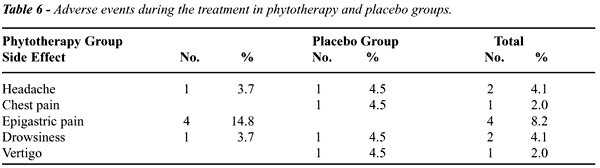
The
percent of adverse events verified during the study was similar between
patients receiving phytotherapics and placebo (Table-6). There was no
predominance of any type of adverse event over other, suggesting that
phytotherapics do not cause any specific adverse event.
DISCUSSION
The
use of phytotherapic agents in clinical management of BPH patients, largely
employed in Europe, has gained popularity in USA in the 90’s, when
there was a rapid increase in clinical use of these drugs (1). According
to Astin (9), reasons contributing to the increase of phytotherapics use
were determined by changes in values, beliefs and orientation of individuals
concerning health and well-being. In addition, these drugs are understood
as more natural, safe, and healthy.
In order to evaluate phytotherapics potential
as alternative for clinical management of BPH, we studied a group of patients
using a combination of 25mg Pygeum africanum and 300mg stinging
nettle extract and compared it to placebo, according to a prospective,
randomized, double-blind protocol, during 6 months. After this period
of treatment we did not find significative differences in clinical improvement
potential between combination of phytotherapics and placebo. Evaluation
of CSR was also similar between the groups, for both clinical and urodynamic
data.
A
multicentric and randomized study, published by Barlet et al. (10) analyzing
263 patients using Pygeum africanum 50 mg bid or placebo during
60 days, showed “voiding improvement” to 66% in PhyG and 31%
in PlaG, and 17.2% and 4.3% increases in Qmax in PhyG and PlaG, respectively.
In this study, the authors demonstrated a clear improvement of Qmax in
PhyG compared to PlaG. However, our data indicate that the placebo effect
may determine increasing of Qmax in up to 13%. Thus, urodynamic effects
of a particular type of clinical treatment for BPH may be considered significative
only if they act in a more consistent manner over the obstructive prostatic
process, what would be demonstrated by a more expressive increase in Qmax.
On the other hand, evaluation of clinical efficiency in that study was
compromised, both for the absence of a validated score system and for
the short period of treatment. In other multicentric study, Breza et al.
(11) reported 40% improvement in IPSS and 18% increase in Qmax for 85
patients treated with 100mg Pygeum africanum during 2 months. In this
study, the absence of a control group limits interpretation of the results
obtained. In addition, 2 months cannot be considered a conclusive period
of treatment. Recently, Chaterlain et al. (12) reported the results of
a study involving 174 patients using Pygeum africanum (50mg bid
and 100mg qd) during 12 months. Initially the patients entered into a
comparative phase, double-blind, during 2 months, where they received
Pygeum africanum 50mg bid or Pygeum africanum 100mg in one
dose. During this initial period, both treatments presented comparable
efficiency. Then the patients started to receive Pygeum africanum
100mg/day in one dose during 10 months. After 12 months of treatment,
there was a 46% drop in IPSS e 15% increase in Qmax. Here, again, the
percent increase of Qmax shows that results obtained in our study are
consistent with that obtained in the literature. The use of an adequate
methodology is crucial for any clinical trial about BPH, because variations
induced by placebo effect may produce clinical improvement in up to 40%
(13). Thus, the absence of a control group limits significantly IPSS improvement
obtained in Chatelain et al. report. Alternatively, difference in IPSS
improvement profile, compared to our clinical trial may be related to
Pygeum africanum extract dose, suggesting that improving Pygeum
africanum dose from 25mg to 100mg may occasionally be translated by
an improvement in clinical efficacy.
The
single recent study about stinging nettle, performed in Germany, involving
41 patients treated during 3 months with a liquid presentation of the
product, showed IPSS improvement superior to placebo (14). However, the
preparation has been removed from the market, because its unacceptable
taste was rejected by patients.
Analysis
of these reports show that most published studies about phytotherapics
present important methodological defects. To better illustrate this scenario,
Andro & Riffaud (15) published a review of 25 years of experience
with Pygeum africanum, where they found 2.262 patients treated
with this extract. Pygeum africanum dose ranged from 50mg to 200mg,
and no study lasted more than 12 weeks. Only twelve of these studies involved
a double-blind, placebo controlled protocol, and just seven presented
Qmax analysis. Results showed wide variations, from absence of urodynamic
effect, to 91% increase of Qmax in patients receiving the drug. As the
majority of the studies reported in this review were done before the 90’s,
none has used a validated scoring system. Thus, none is according to the
norms established by international consensus on BPH (16), what makes comparison
with our data rather difficult. Actually, our study seems to be the first
to use a combination of Pygeum africanum and stinging nettle, 25mg
and 300mg, respectively, for clinical treatment of BPH patients, using
the methodology recommended by the international consensus of BPH.
Adverse
events occurring during the clinical trial were similarly distributed
between PhyG and PlaG, supporting the belief that, if they are not beneficial
in controlled studies, they don’t cause important adverse effects.
Current
research lines adopted identify only 2 mechanisms scientifically proven
through which it is possible to relieve clinically the symptoms from prostatic
obstructive process: decreasing of prostatic smooth muscle tonus, through
blockade of a-1adrenergic receptors, and reduction of prostatic volume
mediated by the inhibition of 5-a reductase (17). Phytotherapic drugs
do not act upon none of these 2 mechanisms and, thus, from the scientific
point of view, should not be considered first line drugs in BPH clinical
management. However, it is worth emphasizing that the use of phytotherapic
agents in BPH clinical approach is widespread. Thus, it is crucial that
we establish a process of patterning to theses drugs, determining the
composition, pharmacokinetics, and mechanism of action involved. Finally,
only through multicentric, prospective, randomized, placebo controlled,
long term studies, involving an adequate number of patients, will we be
able to offer the necessary support for the definition of phytotherapics’
role in BPH.
CONCLUSION
Combination of 25mg Pygeum africanum and 300mg stinging nettle extract produced clinical and urodynamic effects similar to placebo in a group of BPH patients.
REFERENCES
- Lowe FC, Fagelman E: Phytotherapy in the treatment of benign prostatic hyperplasia: an update: Urology 1999; 53:671-8.
- Wagner H, Flachsbarth H, Vogel G: A new antiprostatic principle of stinging nettle (Urtica dioica) roots. Phytomedicine 1994; 1:213-24.
- Hirano T, Homma M, Oka K: Effects of stinging nettle root extracts and their steroid components on the Na+, K--ATPase of the benign prostatic hyperplasia. Planta Med. 1994; 60:30-3.
- Hryb DJ, Khan MS, Romas NA, Rosner W: The effects of extracts of the roots of the stinging nettle (Urtica dioica) on the interaction of SHBG with its receptor on human prostatic membranes. Planta Med. 1995; 61:31-2.
- Lowe FC, Fagelman E: Phytotherapy in the treatment of benign prostatic hyperplasia: an update. Urology 1999; 53:671-8.
- Levin RM, Riffaud JP, Bellamy F, Rohrmann D, Krasnopolsky L, Haugaard N, et al.: Effects of Tadenan® pretreatment on bladder physiology and biochemistry following partial outlet obstruction. J Urol. 1996; 156:2084-8.
- Levin RM, Riffaud JP, Bellamy F, Rohrmann D, Habib M, Krasnopolsky L, et al.: Protective effect of Tadenan® on bladder function secondary to partial outlet obstruction. J Urol. 1996; 155:1466-70.
- Melo EA, Mattos Jr. D, Rios LAS: A double-blind, randomized, placebo-controlled study, to assess the efficacy of alfuzosin in the treatment of patients with benign prostatic hyperplasia. Braz J Urol. 2002; 28:25-32.
- Astin JA: Why patients use alternative medicine: result of a national study. JAMA. 1998; 279:1548-53.
- Bartlet A, Albrecht J, Aubert A, Fischer M, Grof F, Grothuesmann HG, et al.: Efficacy of Pygeum africanum extract in the medical therapy of urination disorders due to benign prostatic hyperplasia: evaluation of objective and subjective parameters. A placebo-controlled double-blind multicenter study. Wien Klin Wochenschr. 1990; 102:667-73.
- Breza J, Dzurny O, Borowka A, Hanus T, Petrik R, Blane G, et al.: Efficacy and acceptability of Tadenan® (Pygeum africanum extract) in the treatment of benign prostatic hyperplasia (BPH): a multi-culture trial in Central Europe. Curr Med Res Opin. 1998; 14:127-39.
- Chatelain C, Autet W, Brackman F: Comparison of once daily and twice daily dosage forms of Pygeum africanum extract in patients with benign prostatic hyperplasia: a randomized, double-blind study, with long-term open-label extension. Urology 1999; 54:473-8.
- Hansen BJ, Meyhoff HH, Nordling J, Mensink HJ, Mogensen P, Larsen EH: Placebo effects in the pharmacological treatment of uncomplicated benign prostatic hyperplasia. Scand J Urol Nephrol. 1996; 30:373-7.
- Engelmann U, Boos G, Kres H: Therapie der benignen Prostathyperplasie mit Bazoton Liquidum. Urologe B. 1996; 36:287-91.
- Andro MC, Riffaud JP: Pygeum africanum extract for the treatment of patients with benign prostatic hyperplasia: a review of 25 years of published experience. Cur Ther Res. 1995; 56:796-817.
- Denis LJ, Connell JMC, Yoshida O, et al: Recommendation of the International Scientific Committee: The evaluation and treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic obstruction, in Proceedings of the 4th International Consultation on Benign Prostatic Hyperplasia (BPH), Paris, July 2–5, 1997. Health Publication Ltd. 1998; pp. 671-2.
- Lepor H: The treatment of benign prostatic hyperplasia: A glimpse into the future. Urol Clin North Am. 1995; 22:455-9.
_____________________
Received: June 24, 2002
Accepted after revision: October 16, 2002
_______________________
Correspondence address:
Dr. Ézer Amoras Melo
Av. Ricardo Jafet, 148
São Paulo, SP, 04260-020, Brazil
Fax: + 55 21 272-0966
E-mail: ezeramelo@uol.com.br